Abstract
This study has been conducted to investigate the effect of incorporating CuO nanoparticles inside PVA matrix with the aim of enhancing the mechanical properties of PVA for reinforcement purposes. Films were prepared by solution intercalation technique with 0.5, 1.0, 1.5 and 2.0 wt% CuO nanofiller content taking neat PVA as the reference for the study. Structural characterisations of the prepared nanocomposite films were carried out by FTIR, SEM and XRD techniques. Various mechanical parameters such as tensile strength, elastic modulus, flexural strength and toughness were investigated and significant enhancement in the properties of polyvinyl alcohol films containing CuO nanofillers was observed. Elastic modulus and toughness showed a linear relation with respect to nanofiller concentration at low wt%, whereas at higher wt% of CuO, the nature was observed to be non-linear. PVA-2.0 wt% CuO nanocomposite showed the highest values of flexural strength, toughness and tensile strength among all the fabricated nanocomposite films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the many unique attributes of polymer nanocomposites, the most noteworthy are their high surface area, high aspect ratio and enhanced mechanical properties. These properties offer tremendous potential for exciting technological innovations and other research areas in the fields of material science. The properties of these composites are dependent upon the inorganic/organic materials reinforced in the polymer matrix [1–6]. Therefore, an important usage of nanomaterials today is in reinforcing polymer composites due to their ultra high tensile strength, stiffness and various other mechanical properties.
Generally, the efficiency of reinforcing fillers in the matrix is inversely proportional to the size of fillers and proportional to the surface-to-volume ratio of nanofiller dispersed in the matrix. Therefore, the geometry of the filler is an important factor, which affects the reinforcement performance of the nanoparticles-filled composites. If the surface-to-volume ratio of the nanofiller is greater, the blending result will become more effective [4–7].
Although a lot of work has been done on polymer composites, the effect of copper oxide nanoparticles as filler materials on properties of composites is yet to be widely explored. In recent years, copper oxide nanostructures have been used extensively in photocatalytic degradation of dyes, in field emission structures [8], sensor applications [9] and other areas of science and technology as well.
This present work deals with the study of effect of copper oxide nanoparticles on mechanical properties of polyvinyl alcohol films. PVA has been chosen as the matrix for nanofiller reinforcement due to its good film-forming ability, biocompatibility, good chemical resistance and interesting mechanical properties [10, 11]. Furthermore, PVA being a hydrophilic polymer facilitates the synthesis of composites with uniform distribution of nanoparticles inside the polymer matrix. The hydroxyl groups in PVA also have a very strong tendency of forming charge-transfer complexes with CuO; therefore, good interaction occurs between the polymer matrix and nanoparticles [12]. CuO has been previously reported as a suitable filler for strengthening the mechanical and physical properties of few powder ceramics and polymeric nanocomposites [13, 14]; therefore, it has been chosen as a nanofiller for synthesis of PVA composites.
In this study, Fourier transform infrared spectroscopy (FTIR) study was performed to identify possible interactions between the polymer matrix and CuO nanoparticles. XRD was performed in order to understand the nanostructured feature and crystallinity of pure CuO and of the prepared nanocomposite films. The surface morphology was investigated using scanning electron microscope (SEM). The mechanical properties of nanocomposites such as tensile strength, flexural strength and toughness were evaluated and discussed. The aim of this study is to investigate the effect of CuO nanoparticles on mechanical properties of PVA polymer for reinforcement purposes and for applications in areas, which demand enhanced mechanical properties such as flexural strength, ultimate tensile strength, toughness and elastic modulus.
Reagents and materials
Copper nitrate trihydrate (≥90 %) and glycine were obtained from M/s. Merck India and M/s. SD Fine Chemicals, respectively. PVA (polyvinyl alcohol) was obtained in powder form from M/s.SD Fine Chemicals having an average molecular weight of 124,000 (86–89 % hydrolysed). Distilled water was used for dissolution and PVA film casting.
Synthesis of CuO nanoparticles
CuO nanoparticles were prepared by glycine nitrate combustion method. Copper(II) nitrate trihydrate [Cu(NO3)2·3H2O] was used with glycine (CH2NH2COOH) where glycine was used as a fuel. The fuel-to-nitrate (F/N) ratio was kept constant at 0.3. As per the maintained ratio, 19.372 g of copper nitrate trihydrate and 1.8 g of glycine were dissolved in 125 ml of distilled water. The reaction mixture was placed on a hot plate. As the heating progressed, water vapour, carbon dioxide gas and nitrogen dioxide gas (characterised by reddish brown color and pungent odour) were released resulting in the formation of gel. The reaction was completed by self-ignition (combustion) minutes after the formation of gel, leaving behind a highly fine, porous powder. The obtained product was then calcined at 400 °C to produce nanopowder of CuO. The stoichiometry of the reaction is as follows:
From the reaction it is clear that 76 mol of gases/13 mol of CuO are released during the reaction. The liberation of gaseous products during combustion reaction increases the surface area of the powder by creating micro and nanoporous regions.
Fabrication of PVA-CuO nanocomposites
PVA-CuO nanocomposites were prepared by solution intercalation film casting method. Films were cast from a CuO water suspension, in which PVA was dissolved by heating at 65 ± 5 °C for 4 h with constant stirring on a water bath. The solid content of the solution was maintained at 7.5 % by weight. The solution was then ultrasonicated at 65 ± 5 °C for 45 min, poured into a clean glass mould and then dried at room temperature for 48 h. The PVA nanocomposite films were cast with varying amounts viz., 0.5, 1, 1.5 and 2 % of CuO nanoparticles. The prepared films were free from air bubbles with uniformly dispersed CuO nanoparticles in the PVA matrix. The thickness of the nanocomposite film samples ranged from 0.19 to 0.21 mm. The thickness of the films was measured using LCD digital vernier callipers.
Characterisations of nanocomposite films
For investigating the mechanical properties of composites, it is essential to understand the interaction of CuO nanofiller with the PVA matrix as well as some insight on the surface morphology of PVA-CuO nanocomposite films would be beneficial in knowing the effect of filler addition in PVA polymer matrix. For this purpose, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and scanning electron microscopy (SEM) studies were performed and some interesting results were observed.
Fourier transform infrared spectroscopy
FTIR spectra of the samples was measured by plotting a graph of %transmittance versus wavenumber (cm−1) in the spectral range of 4000–500 cm−1 using Bruker Alpha Fourier transform-infrared spectrometer (FTIR) with resolution of 4 cm−1. FTIR analysis was performed to understand possible interaction between PVA matrix and CuO nanoparticles. The FTIR spectra of PVA and CuO filled PVA are shown in Fig. 1. A strong broad absorption band at 3000–3300 cm−1 is assigned to O–H stretching vibration of PVA. The band corresponding to C–H asymmetric stretching vibration occurred at 2914–2925 cm−1. A peak at 1713–1736 cm−1 corresponds to C=O stretching vibration (of vinyl acetate group of PVA). A peak at 1416–1431 cm−1 is due to C–H bending of –CH2 in PVA back bone. A band at 1300–1332 cm−1 corresponds to –CH2 wagging. Another strong band observed at 840–845 cm−1 has also been attributed to –CH2 in stretching mode. A band at 1080–1090 cm−1 is attributed to C–O–C stretching of acetyl group present on PVA backbone. There was no appearance of additional peak in IR spectra; however, a considerable shift in the peak position of the band corresponding to C=O stretch and C–O–C stretch vibrations indicated a physical interaction between hydroxyl groups of PVA and nano CuO. The hydroxyl groups of PVA have a very strong tendency to form charge-transfer complex with CuO nanoparticles through chelation [10].
X-ray diffraction analysis
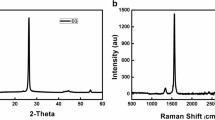
The X-ray diffraction measurements were performed to examine the nanostructured feature and crystallinity of pure CuO and PVA-CuO polymer nanocomposites. X-ray’s diffraction (XRD) analysis was recorded using Rigaku Smartlab advanced wide-angle X-ray diffractometer. The X-ray source was Ni-filtered Cu-Kα radiation (40 kV, 30 mA). The dried membranes of uniform thickness (200 μm) were mounted on a sample holder and the X-ray tracings were recorded in the range of 0°–80° at a speed of 8° per min. The crystalline nature of pure nano CuO was observed by various sharp peaks shown by XRD patterns in Fig. 2a. Ten distinct diffraction peaks can be seen at the 2θ values of 32.56°, 35.59°, 38.82°, 48.24°, 53.50°, 58.41°, 61.56°, 68.1°, 72.42° and 75.28° for the nano sized crystalline monoclinic phase of CuO and the values were found to be in good agreement with literature values (JCPDS file no. 48–1548).
XRD patterns of pure polyvinyl alcohol and PVA-CuO nanocomposites having 0.5, 1, 1.5 and 2 wt% of CuO, respectively, are shown in Fig. 2b. All the composites showed a peak at approximately 2θ = 19.4° corresponding to (1 0 1) crystal plane for polyvinyl alcohol, which indicates the semi-crystalline nature of PVA. The crystalline nature of PVA results from the strong intermolecular interaction between PVA polymer networks. XRD profiles of the composites showed no shifting or broadening of peak at 2θ = 19.4°, but showed appearance of smaller new peaks at 32.56°, 35.59°, 38.82°, 48.84°, 53.50° and 58.56° for nanosized CuO which clearly indicated the presence of CuO nanoparticles inside the PVA matrix [10]. Since PVA contains a large number of hydroxyl groups, it can effectively inhibit the aggregation of CuO nanoparticles and helps to keep the nanoparticles well dispersed in the aqueous PVA solution at the nanoscale level.
Scanning electron microscopy (SEM)
Assessment of nanoparticles dispersion and morphology was understood by the SEM photomicrographs of the nanocomposites. Surface images were recorded by scanning electron microscopy (SEM, Carl Zeiss Ultra 55 Model) at an operating voltage of 5 kV. Figure 3a, b shows SEM images of the synthesised CuO nanopowder at the micrometre and nanometre scale, respectively. Figure 3a shows that the synthesised nanopowder is highly porous and voluminous consisting of agglomerated spherical nanoparticles. The agglomerated nanoparticles are observed to be of sizes in the range of 80–120 nm as shown by Fig. 3b.
Figure 4 shows the SEM images of (a) neat PVA, (b) PVA-0.5 % CuO, (c) PVA-1.0 % CuO, (d) PVA-1.5 % CuO, (e) PVA-2.0 % CuO. From these images, it was observed that the addition of CuO nanoparticles in PVA polymer exhibited changes in the surface morphology of composite films. The white spots shown in Fig. 4 are CuO nanoparticles. From the figures, the particle sizes were seen to increase with increase in filler concentration which clearly suggests that particle agglomeration takes place at higher nanofiller concentrations. The different obtained sizes may be possible due to agglomeration of the nanoparticles which takes place in order to minimise the high surface energy that exists in individual nanoparticles. The smallest particle size obtained from the image was 44 nm while the largest particle size obtained due to agglomeration was 240 nm.
Mechanical properties analysis
The mechanical properties of PVA-CuO nanocomposite films and neat PVA were measured by KIPL-PC 2000 UTM (universal testing machine) with tensile flat surface grips at a test speed of 10 mm/min. The tensile tests were performed in accordance with ASTM D638 standards. The load cell was set at 0.2 kN. The prepared films were 0.19–0.21 mm thick and had widths in the range of 17–20 mm. The testing machine had an error range of ±0.2 MPa.
Results and discussions
PVA is a water-soluble polymer and hence facilitates the synthesis of composites with uniform distribution of nanoparticles. The maximum amount of nanofiller content was 2.0 wt% since higher levels of filler loading would only cause agglomeration of nanoparticles thereby affecting the properties and the surface area of composites. Different mechanical properties such as ultimate tensile strength (UTS), toughness, flexural strength, elastic modulus (E) and stress/strain relationships were determined as a function of filler content in the polymer matrix.
Variation of stress/strain relationship with filler content
Figure 5 shows engineering stress/strain curves for neat PVA and prepared nanocomposite films, respectively. From Fig. 4 and Table 1, it was observed that with increasing CuO filler content, higher levels of stress at break could be achieved as compared to that of neat PVA. The observed results suggest that the fine copper oxide nanoparticles would reinforce themselves in the PVA matrix and get oriented along the direction of stress. The stress/strain curves also suggest an increase in elongation at break values of PVA with the incorporation of CuO nanoparticles. This result indicates that the incorporation of nanoparticles would improve the molecular interaction inside the polymeric matrix. Increased bonding through chelation would take place between the hydroxyl groups of PVA and copper oxide nanoparticles at higher nanofiller content thus leading to enhanced elongation at break values as well as ultimate stress values.
Filler effect on elastic modulus
Figure 6 shows variation of elastic modulus with CuO nanofiller content. The variation observed was linear in nature at low CuO content and deviation from linearity was observed at higher CuO content of 1.5 and 2.0 wt%The results observed are clearly shown in Table 1. It was observed that the elastic modulus values increased with the addition of CuO nanoparticles and the highest elastic modulus was observed at 2.0 wt% CuO/PVA nanocomposite specimen. This suggests that the incorporation of nanoparticles in the polymer matrix would improve the stiffness of PVA. Strong interfacial interaction will enable the load to be transferred easily across the nanoparticle-matrix interface.
Tensile strength properties
Figure 7 shows variation of ultimate tensile strength with CuO filler content. In this study, engineering ultimate tensile strength values have been reported. It was observed that with increasing filler content, the ultimate tensile strength values increased significantly and showed an increase of 49.25 % between neat PVA and PVA/2 wt% CuO composites. This may be possible due to strong reinforcement of nanofillers inside the matrix due to which efficient load transfer may take place from the matrix to the fillers across the polymer-matrix interface.
Toughness properties
Total area under a stress/strain curve is indicative of overall toughness and is a measure of the amount of energy required to break a specimen. Remarkable enhancement in toughness of PVA-CuO nanocomposite film was reported in Table 1 and Fig. 8. The increase in toughness of 2 wt% CuO/PVA film was 172.23 % more than that of neat PVA. The enhanced toughness may be attributed to structural and morphological changes induced by the nanoparticles, as well as their increased mobility compared to micrometer-size fillers. The highest toughness was showed by 2.0 wt% CuO filler containing composite film.
Flexural strength properties
Figure 9 and Table 1 show the effect of CuO loading on flexural properties of PVA. The observed change was not significant at low nanofiller content; however, 53.28 % rise in flexural strength of PVA was observed at CuO loading of 2 wt% in the matrix. This may be due to enhancement of various factors such as degree of dispersion of filler, adhesion at filler-matrix interface and also the aspect ratio of the filler with increasing filler content. The flexural strength of the composites depends on these factors to a great extent.
Conclusion
Polyvinyl alcohol was taken as a matrix for incorporating copper oxide nanoparticles as nanofillers by solution intercalation film casting method. The nanofiller content was varied from 0 to 2 % in increments of 0.5 % in the composite films by weight. The surface morphology and interactions of the polymer matrix-nanoparticles was studied using SEM, XRD and FTIR techniques. Remarkable improvements in various mechanical properties such as elastic modulus, toughness, flexural strength, tensile strength were observed with reinforcement of CuO nanofillers in the polymer matrix. The results are tabulated and shown in Table 1. The best mechanical properties were observed at CuO loading of 2.0 wt% where an almost 50 % increase in ultimate tensile strength was observed as compared to neat PVA and the flexural strength increased by 53 % at the same loading. Toughness of PVA composite film containing 2.0 wt% of CuO increased by almost 173 % and was reported as the highest among all specimens. In any case, our results suggest that it would be suitable to explore further mechanical properties of composites with such reinforcements. Such nanocomposites may be promising candidates for applications in nanotechnology in future.
References
Ching YC, Yaacob II (2012) Effect of polyurethane/nanosilica composites coating on thermo-mechanical properties of polyethylene film. Mater Technol 27:113–115
Ching YC, Chen CY, Yaacob II (2012) Weathering resistance of solventborne polyurethane/nanosilica composite. Adv Sci Lett 12:165–169
Lagashetty A, Venkataraman A (2005) Polymer nanocomposites. Resonance 10(7):49–57
Chee CY, Yaacob II (2010) Influence of nanosilica/polyurethane composite coating on IR effectiveness and visible light transmission properties of polyethylene. Adv Mater Res 97–101:1669–1672
Li S, Zhang S, Zhou S, Gu G, Wu L (2011) Physical and optical properties of silica/polymer nanocomposite inverse opal. Adv Sci Lett 4:3445–3450
Chee CY, Yaacob II (2009) Influence of nano-SiO2/polyamide composites coating on thermic effect and optical properties of polyethylene film. Int J Mod Phys B 23:1395–1400
Chou CJ, Read AE, Garcia-Meitin EI, Bosnyak CP (2002) Polymer nanocomposite. The Dow Chemical Company, Midland
Hsieh CT, Chen JM, Lin HH, Shih HC (2003) Synthesis of well-ordered CuO nanofibers by a self-catalytic growth mechanism. Appl Phys Lett 82:3316
Zhang J, Liu J, Peng Q, Wang X, Li Y (2006) Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem Mater 18:867–871
Shao C, Kim HY, Gong J, Ding B, Lee D, Park S (2003) Fiber mats of poly (vinyl alcohol)/silica composite via electrospinning. Mater Lett 57(9):1579–1584
Goodship V, Jacobs D (2009) Polyvinyl alcohol: materials, processing and applications. Rapra Rev Rep 16(12). Smithers Rapra Technology, Shrewsbury, Shropshire. ISBN 9781847350954
Rashmi SH, Raizada A, Madhu GM, Kittur AA, Suresh R, Sudhina HK (2015) Influence of zinc oxide nanoparticles on structural and electrical properties of polyvinyl alcohol films. Plast Rubber Compos 44(1):33–39
Ramesh S, Gill C, Lawson S (1999) The effect of copper oxide on sintering, microstructure, mechanical properties and hydrothermal ageing of coated 2.5Y-TZP ceramics. J Mater Sci 34(22):5457–5467
GuO Z, Liang X, Pereira T, Scaffaro R, Hahn HT (2007) CuO nanoparticle filled vinyl ester resin nanocomposites: fabrication, characterization and property analysis. Compos Sci Technol 67(44):2036–2044
Acknowledgments
The authors would like to acknowledge MSRIT Alumni Association for providing adequate funds for carrying out this study. The mechanical properties of the specimens were analysed using KIPL-PC 2000 UTM (universal testing machine) at The Energy and Resources Institute (TERI) in Bangalore. A portion of this research was also performed using facilities at CeNSE, funded by Department of Information Technology, Govt. of India, located at Indian Institute of Science, Bangalore. The authors are also highly grateful for all the guidance and the technical assistance provided to them by the Department of Chemical Engineering at MSRIT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, J.K., Raizada, A., Ganguly, D. et al. Enhanced mechanical properties of polyvinyl alcohol composite films containing copper oxide nanoparticles as filler. Polym. Bull. 72, 2033–2047 (2015). https://doi.org/10.1007/s00289-015-1386-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1386-4