Abstract
One of the advances in biotechnology has been the development of the capability to produce large quantities of highly purified polypeptides and proteins. Unfortunately, the circulatory half-lives of many of these agents are short, usually of the order of minutes and the time required for a response in tissues is usually long compared to the half-life. Hence, there is always demand for polymeric systems which can deliver the proteins for prolonged period and also to protect the molecules from degradation. The present work was attempted to develop heparin-functionalized gelatin microspheres (HMS) to deliver heparin-binding growth factors particularly for wound-healing applications. The heparin conjugation was carried out using EDC/NHS coupling protocol. Heparin-binding EGF-like growth factor (HB-EGF) was loaded in HMS and its in vitro release behaviour in an environment with or without proteases was studied. The bioactivity of the HB-EGF released from the microspheres was assessed using NIH 3T3 mouse embryonic fibroblast culture. The extent of heparin modification was found to be 1.97 μmol/g of HMS and demonstrated significant protection against enzymatic degradation and sustained release of HB-EGF for more than 10 days. The bioactivity of HB-EGF released from the HMS was retained during the observed release period. The HMS was also found to be non-toxic as determined by calcein AM fluorescent staining. The overall study suggests that the HMS could be used as a growth factor’s delivery component in tissue engineering scaffolds particularly for wound-healing applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Normal response to tissue injury is a compilation of sequential and overlapping events involving several cell types and growth factors that ideally result in the restoration of biological and functional integrity and ultimately ending with wound repair and closure. However, in a person with diabetes, the healing process is impaired and takes more time even if the injury is minor and often leads to diabetic ulcers. Current treatment for such wounds or ulcers includes the administration of antimicrobials followed by application of growth factors containing gels around the lesions [1].
The polypeptide growth factors are a class of biological mediators that have diverse effect on cell proliferation, motility, matrix synthesis and differentiation. Several investigators have shown that the level of growth factors is markedly decreased in chronic wounds compared to acute wounds [2, 3]. It is postulated that repeated trauma and infection increase the presence of pro inflammatory cytokines with subsequent increase in the level of matrix metalloproteinase (MMPs) leading to the degradation of growth factors. However, it has been shown that venous stasis ulcers are not absolutely deficient in growth factors, but the growth factors appear to be trapped within the fibrin cuffs surrounding capillaries [4, 5]. Several other investigators have also suggested that the trapping occurs in diabetic ulcers of the lower extremity [6]. These above factors suggest a potential role for exogenous growth factors in treating chronic wounds.
Numerous growth factors, which are thought to have a potential role in wound healing such as platelet derived growth factor (PDGF), epidermal growth factor (EGF), basic fibroblasts growth factor (bFGF) and vascular endothelial growth factor (VEGF) have been isolated, cloned, produced as recombinant molecules and evaluated for their potential to accelerate wound healing [7]. However, to date only PDGF has been approved by the FDA for use in diabetic ulcers [8]. Preliminary reports show that the conventional delivery systems used in clinical trials are ineffective due to the high level of MMPs in the chronic wound. Over the past few decades, several technologies have been developed to deliver growth factors by incorporating them into hydrogels [9] micro and nanoparticles [10], and so on. However, bioavailability of growth factors is very poor, since they have a short half-life and rapid clearance from the implant site. The development of a delivery system, which exhibits greater entrapment efficiency with controlled release of growth factors in an active form, would have a significant clinical impact in the field of tissue engineering.
Several of these growth factors have been shown to bind to heparin and this binding stabilizes and protects these growth factors from inactivation and degradation [11]. Based on this, several heparin-containing systems have been developed for the controlled release of the growth factors. Wissink et al. [12] modified the collagen matrices by covalently conjugating heparin for physical binding of bFGF. The alginate gel covalently cross-linked with heparin was demonstrated for sustained release of bFGF [13]. Heparin-functionalized PLGA nanoparticles were reported by Chung et al. [14] who demonstrated the sustained release of VEGF. The long-term delivery of heparin-binding growth factors such as bFGF was also demonstrated by heparin-conjugated polymeric micelle [15]. However, none of the heparin-functionalized delivery systems have been demonstrated for its potential effect in chronic wound healing. Hence, it is proposed that the application of heparin-functionalized delivery systems to deliver the heparin-binding growth factors particularly to address chronic wounds would be the novel approach and would have high impact in chronic wound-healing therapy.
Gelatin a natural polymer has long history of medical use for designing wound dressing materials, tissue engineering scaffolds, drug delivery matrices, hydrogels [16] and microspheres [17]. It is biodegradable, biocompatible, non-immunogenic and suitable for easy modification of the functionality of the molecules [18, 19]. Gelatin has been exploited extensively for growth factor delivery particularly for bFGF and it was believed that the electrostatic interaction sustained the release of the bFGF [20]. In our previous report aminated gelatin microspheres were prepared and functionalized with a MMP inhibitor dihydroxybenzoic acid and demonstrated its potential for inhibition of MMPs from diabetic wound tissue [21]. In the present study, attempt was made to functionalize the aminated gelatin microspheres with heparin using EDC/NHS coupling protocol and evaluated for its potential as drug delivery systems for growth factors. Heparin-binding EGF-like growth factor (HB-EGF), a polypeptide chain of 87 amino acid residue, demonstrated for wound healing [22] was used as a model growth factor. The developed system was studied for its potential against enzymatic degradation, entrapment efficiency and controlled release of growth factors in an environment with or without proteases.
Materials and methods
Materials
Gelatin Type B from bovine skin (bloom strength ~ 225), soya oil, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), ethylenediamine (ED), morpholinoethanesulphonic acid (MES), heparin sodium salt from porcine intestinal mucosa (average MW ~ 3,000), collagenase crude Type 1A from Clostridium histolyticum, Dulbecco’s modified eagle’s medium (DMEM), Foetal bovine serum and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma, St. Louis, USA. Human recombinant HB-EGF and Human HB-EGF Duoset ELISA kit was obtained from R&D systems, Minneapolis, USA. NIH 3T3 mouse embryonic fibroblast cell line was obtained from NCCS, Pune, India. All other reagents were of analytical grade, obtained from SD fine chemicals Mumbai, India.
Preparation of cross-linked gelatin microspheres
Gelatin microspheres were first prepared by emulsification solvent extraction method and then cross-linked using 50 mM EDC as reported earlier [23]. Briefly, gelatin aqueous solution (10 ml of 15 % w/v) was added at 60 °C to 100 ml of soya oil pre-heated to 60 °C. The two phases were emulsified for 5 min using overhead stirrer (Remi Instruments, Mumbai, India) rotating at about 400 rpm. The emulsion was then rapidly cooled to 5 °C in an ice bath and stirring continued for 30 min to allow the spontaneous gelation of the gelatin aqueous solution. The microspheres formed were dehydrated by stirring with pre-cooled acetone, recovered by filtration, washed several times with acetone and vacuum dried for at least 2 days. The dried microspheres (500 mg) were suspended in 25 ml of acetone: water (8:2) containing EDC (50 mM) and stirred using magnetic stirrer for 24 h at 5 °C. Microspheres were then filtered and washed extensively with deionized water and then with acetone and vacuum dried for at least 2 days.
Preparation of heparin-modified gelatin microspheres
The cross-linked microspheres were aminated using ethylenediamine and the amine-terminated microspheres were then conjugated with the carboxylic acid groups of the heparin using EDC/NHS coupling protocol.
Preparation of aminated gelatin microspheres
Aminated gelatin microspheres were prepared by conjugating ethylenediamine with cross-linked gelatin microspheres using EDC coupling protocol as reported earlier [21]. Briefly, cross-linked gelatin microspheres (CMS) (1 g) were dispersed in 100 ml of 0.1 M MES buffer containing 1 N NaOH at pH 5.0. EDC (50 mM) and ED (2 g) were added and allowed to stir at room temperature in a magnetic stirrer for 3 h and the pH was maintained at 5.0 by adjusting with 1 N HCl or 1 N NaOH accordingly. The reaction mixtures were then filtered and the microspheres were suspended in a 0.1 M Na2HPO4 and 2 M NaCl solution for 1 h with moderate stirring. Microspheres were then filtered and washed extensively with deionized water for 20 min, followed by dehydration with 50 % acetone and finally with neat acetone. The aminated microspheres (AMS) thus obtained were vacuum dried for at least 2 days.
Heparin modification of AMS
Heparin at various concentrations as given in Table 1 was dissolved in 0.1 M MES buffer (pH 5.5). EDC (50 mM) and NHS (50 mM) were added and stirred 15 min under magnetic stirrer at room temperature for pre-activation of COOH groups of heparin. AMS was then dispersed into the activated heparin solution and stirring continued for 3 h at room temperature and the pH was maintained at 5.5. The heparin-modified microspheres (HMS) were recovered by filtration and washed extensively with deionized water for 20 min, followed by dehydration with 50 % acetone and finally with neat acetone. The obtained HMS was then vacuum dried and stored in desiccators until use. The degree of heparin modification was determined by performing toluidine blue assay.
Localization of immobilized heparin
Alcian Blue staining was used for localization of immobilized heparin in HMS. Microspheres were treated with Alcian Blue 8GX (0.5 % w/v) in 3 % acetic acid solution for 30 min. After washing with water, the microspheres were dried under vacuum and examined under microscope (Leica trinocular light microscope) for the colour development.
Toluidine blue assay
The amount of heparin conjugated to the microspheres was determined by the toluidine blue assay as reported elsewhere [24]. Toluidine blue (50 mg) was dissolved in 0.01 N HCl containing NaCl (0.2 wt%). A known amount of HMS was weighed and dispersed in 2.5 ml of NaCl (0.2 wt%) and to this was added 2.5 ml of toluidine blue solution, the mixture was agitated with a vortex mixer. Normal hexane (2.5 ml) was added and the mixture was shaken vigorously, so that the toluidine blue–heparin complex could be extracted into the normal hexane layer. The hexane layer was then removed and the aqueous solution containing microspheres was centrifuged at 5,000 rpm for 5 min. The remaining toluidine blue from the supernatant was determined by measuring the absorbance at 631 nm, after diluting the solution into 10 fold with NaCl (0.2 wt%), using PerkinElmer lamda 45 spectrophotometer. The concentration of conjugated heparin was calculated, based on calibration with reference standard.
Morphological analysis
The morphology of the microspheres was assessed by scanning electron microscope (JEOL-JSM-5200). The samples were mounted on aluminium stubs using a double-side adhesive tape and sputter coated with gold. Particle size measurements of the gelatin microspheres were performed at least five times with the use of Malvern diffraction particle size analyser (Malvern Mastersizer E-Laser, UK). The particles were analysed at a focal length of 300 mm using isopropanol as a non-reacting dispersion medium. The samples were kept at constant stirring using a magnetic cell stirrer till completion of analysis.
In vitro enzymatic degradation
Enzymatic degradation of the HMS was carried out using collagenase crude type 1A from Clostridium histolyticum with an activity of 441 U/mg. An exactly weighed amount of microspheres (100 mg) was suspended in 5 ml of Tris buffer, pH 7.4 with or without collagenase (20 U/ml) and incubated at 37 °C for 72 h. EDTA (10 mM) in water was then added to prevent further degradation and then filtered. The residue was washed with water and oven dried at 105 °C until constant weight was reached. The % weight loss of the microspheres after enzymatic degradation was quantified as follows
where W 1 is the initial weight of the microspheres and W 2 is the weight of microspheres after enzymatic treatment.
Growth factor entrapment
To the heparin-modified gelatin microspheres (100 mg) about 100 μl of HB-EGF in phosphate-buffered saline (PBS) (1 mg/ml) was added and vortexed for 30 min in cold room (4–8 °C) and then vacuum dried. The HB-EGF-loaded microspheres were then stored under desiccation in cold room.
In vitro release studies
A known amount of microspheres were suspended in 1 ml of PBS with or without Collagenase Type I A (20 U/ml) in 1.5 ml Eppendorf tube and incubated at 37 °C with mild shaking. At every 24 h, the suspension was centrifuged at 5,000 rpm for 5 min and the supernatant was recovered and stored at −20 °C. A fresh release medium (1 ml) was added and the whole process was continued for 10 days. The amount of HB-EGF released was estimated by ELISA.
In vitro bioactivity of HB-EGF
The mitogenic potential of the standard HB-EGF and HB-EGF released from microspheres in presence of proteases was evaluated by performing MTT assay using NIH 3T3 mouse embryonic fibroblasts. Cells were routinely cultured as monolayers in 25 cm2 culture flasks using Dulbecco’s modified Eagles medium (DMEM) with 10 % foetal bovine serum (FBS). For cell growth assay, cells were seeded in 96-well plates at 10,000 cells/well containing DMEM with 10 % FBS and allowed to attach and maintained for 16 h. Cells were then washed with PBS and treated with serum-deprived DMEM for 24 h. After 24 h, the medium was replaced with fresh serum-free medium containing standard HB-EGF at various concentrations such as 0.5, 1.0, 5.0, 10 and 100 ng/ml. About 10 ng/ml equivalent HB-EGF (determined by ELISA) present in the release medium on day 1 (HMS 1 and CMS 1), 5 (HMS 5 and CMS 5) and 10 (HMS 10 and CMS 10) was used. The control was devoid of either serum or growth factors. Cells were then incubated at 37 °C in 5 % CO2 and 95 % air atmosphere for 24, 48 and 72 h. After the incubation period, 10 μl of MTT aqueous solution (5 mg/ml) was added to each well and incubated at 37 °C for 4 h. After removal of the supernatants, the purple formazan crystals formed were dissolved in 200 μl of dimethylsulphoxide and the absorbance [A] was measured using Biorad (Model 680) microplate reader at 570 nm. The relative cell growth (%) related to control cells containing without HB-EGF was calculated from the following equation
Cytotoxicity
The cytotoxicity of the HMS was evaluated using NIH 3T3 mouse embryonic fibroblasts. Cells were seeded in 24-well plates (4 × 104 cells/well) containing DMEM with 10 % FBS and allowed to attach and maintained for 16 h. Then the medium was replaced with DMEM with 1 % FBS containing 1 mg of HMS. The viability of the fibroblasts in presence of the microspheres was observed by fluorescence microscope using a fluoroprobe calcein AM [23]. After treating the cells with microspheres for 72 h, 0.2 μM of calcein AM was added to each well and incubated for 20 min and then viewed under phase contrast fluorescent microscope (Leica DMIRB, USA). Photomicrographs were taken to visualize the metabolically active cells.
Statistical analysis
All quantitative data were expressed as the mean ± standard error. Statistical analysis was performed by unpaired Student’s t test using GraphPad Prism software. A value of p < 0.05 was considered statistically significant.
Results and discussion
Preparation of heparin-modified gelatin microspheres
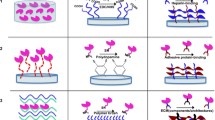
An attempt was made to functionalize the gelatin microspheres with heparin by conjugating the COOH group of heparin with the NH2 groups of gelatin. As the cross-linked microspheres possess very limited number of NH2 groups (7.0 mol/g), as they are utilized in microspheres cross-linking [23], the heparin conjugation was performed in two steps. First the microspheres were aminated by conjugating with ethylenediamine (ED) and the resulting aminated gelatin microspheres (AMS) were allowed to conjugate with heparin using EDC/NHS as a coupling agent. The aminated microspheres displayed about 27 mol of NH2 groups per gram of AMS and this reveal that about 20.0 mol of ED conjugated per gram of AMS [21].
Throughout the study, it was observed that the addition of NHS did not exert any influence in the activation of carboxylic acid groups present in the gelatin microspheres, during both the cross-linking and conjugation reaction. However, while activating the COOH present in the heparin; it was observed that EDC alone could not favour the conjugation reaction. This is because the rapid hydrolysis of the intermediate O-acylisourea group leads to the reformation of COOH groups. The use of NHS along with the carbodiimide gives activated carboxylic groups which are less susceptible to hydrolysis [25, 26]. Hence, the combination of carbodiimide with NHS is considered for effective conjugation reactions.
Heparin at various weight ratios with respect to the AMS as given in the Table 1 was used to determine the optimal concentration for effective heparin modification. The extent of heparin modification was determined by toluidine blue assay. Since heparin forms complex with toluidine blue, the unbound dye was estimated by measuring absorbance at 631 nm. The addition of NHS along with EDC demonstrated the threefold enhancement in heparin conjugation. The maximum heparin conjugation was found at the weight ratio 4:2 of AMS and heparin, respectively, displayed about 1.97 μmol of heparin per gram of HMS. Further increasing the concentration of heparin did not have any impact on heparin modification and this may be due to the steric hindrance of the heparin molecules. Hence, the weight ratio 4:2 of AMS and heparin, respectively, along with the coupling agents EDC and NHS at 50 mM each was considered optimal and used for further modification of the microspheres with heparin.
The proposed reaction scheme for heparin modification was presented in Fig. 1 and the reaction involves the EDC-mediated activation of COOH groups in gelatin microspheres and subsequent nucleophilic attack resulted in the formation of amide bond for both cross-linked and aminated gelatin microspheres. Further the activation of carboxyl groups at heparin molecule by EDC and NHS, followed by nucleophilic (AMS) attack, resulted in the formation of peptide bond between COOH groups of heparin and NH2 groups of AMS.
Localization of immobilized heparin
Alcian blue is a cationic dye which binds with glycosaminoglycans and this property has been employed in quantitative determination of glycosaminoglycans in solution and selective staining of histological sections [27]. This phenomenon has been used to stain the HMS and observed under microscope. The light microscopic images as shown in Fig. 2 revealed that the Alcian Blue-stained HMS displayed homogenous staining of blue colour through the entire microspheres, whereas the CMS showed no staining using the same procedure. This indicates that the heparin is immobilized homogenously throughout the entire surface of the HMS.
Morphology of HMS
The particle size of the HMS was found to be in the range of 60–130 μm and it was slightly lower than the size of the CMS (80–150 μm), as observed in our previous report, [21] probably due to erosion of microspheres during coupling reactions. Both light microscopy (Fig. 2) and scanning electron microscopy (Fig. 3a) revealed that the microspheres were spherical, uniform with smooth surface. SEM image of the HB-EGF-loaded HMS in Fig. 3b demonstrated that the surface of the microspheres is rough due to the binding of the HB-EGF molecule over the surface. However, HMS demonstrated the lose of sphericity due to surface erossion (Fig. 3c) and partial degradation (Fig. 3d) after HB-EGF release for 10 days in absence and presence of proteases respectively.
In vitro enzymatic degradation
The effect of enzymatic degradation on HMS was studied using a crude mixture of proteases for 72 h. Collagenase crude Type 1A from Clostridium histolyticum at 20 U/ml was used and this contains a mixture of collagenases, gelatinase and minor amounts of other proteases. The weight loss with collagenase degradation was significantly greater than that without collagenase degradation for both CMS and HMS. It was reported that bacterial collagenase splits tropocollagen molecules into small fragments between X and Gly in the Pro-X-Gly-Pro-Y sequence [28]. The weight loss of microspheres without collagenase was very minimal (2–6 %) and this may be due to desorption and surface erosion of the microspheres during 72 h incubation. However, differences in % weight loss were observed between CMS and HMS as seen in Fig. 4. The CMS displayed significantly greater % weight loss than the HMS in both the conditions with or without proteases. This clearly indicates that the HMS displayed significant protection against the proteases when compared to CMS. This is mainly attributed to the presence of heparin in the HMS which may hinder the accessibility of the gelatin molecule to the enzyme and also the additional cross-links which may be exerted during the subsequent conjugation reaction [29].
Growth factor entrapment
One of the biggest problems of protein releasing systems is the loss of the activity that results from the denaturation and deactivation of the protein during the formulation process [30]. In view of this, first the heparin-functionalized carriers were developed and then the swellability of the microspheres in aqueous solution was used to entrap the growth factors. The amount of growth factor to be entrapped was reconstituted in a volume of phosphate-buffered saline which was much less than the volume required to fully impregnate the microspheres. Thus, the solution containing HB-EGF was completely absorbed by swelling process of the HMS and ensured the achievement of 100 % entrapment.
In vitro release
The release of HB-EGF from the microspheres was studied under conditions that somewhat mimicked potential in vivo situations. Since the non-healing wound environment may be expected to secrete more collagen degrading enzymes, i.e. MMPs, we investigated HB-EGF release at relatively high concentration of such proteinases. Subsequent release experiments were performed in the presence of 20 U/ml of collagenase corresponding to 45 μg/ml.
The cumulative release of HB-EGF from CMS and HMS in the presence and absence of collagenase was presented in Fig. 5. There were differences in the release pattern with respect to the presence of collagenase and heparin modification. In the presence of collagenase (CMS-C, HMS-C), the release profiles were characterized by substantial burst phase (40–58 %) and subsequent rapid release (75–90 %) when compared to the corresponding CMS and HMS which displayed small burst phase (18–34 %) and a subsequent slower release (59–82 %). It is postulated that the burst phase is mainly due to the release of the HB-EGF molecule present near the surface of the microspheres by simple diffusion which is possibly adsorbed into the gelatin molecule. The substantial burst phase in presence of the collagenase may be due to the superficial degradation of the gelatin molecule leaving the microspheres structure intact with the release of HB-EGF which is bound to the gelatin molecule. This non-specific binding of growth factors with collagenous matrix was already proposed as a means for a controlled release by Kanematsu et al. [31]. However, in HMS, the procedure for covalently incorporating the heparin into microspheres leads to an additional cross-linking of the microspheres and brings about an increase in the resistance against the in vitro degradation and slows down the release when compared to the CMS. The subsequent slow phase release is mainly attributed to release of HB-EGF molecules, which are physically bound to the covalently incorporated heparin. Since the affinity of HB-EGF with heparin is very high, the release may be associated with the surface erosion and partial degradation [14, 29]. In presence of collagenases, the rate of degradation of HMS was accelerated as seen in the SEM image of HMS (Fig. 3d) and results in the increased rate of HB-EGF release and accounts for about 75 % release after 10 days. Whereas in absence of collagenase, the HMS displayed only 59 % release of HB-EGF in the second phase of slow release. The study reveals that the release profile depends on both the degree of heparin modification and presence of proteases.
In vitro bioactivity of HB-EGF
HB-EGF is a mitogenic polypeptide, present in many mammalian species in a variety of tissues and wound fluids, which can stimulate the proliferation of epithelial cells, fibroblasts and smooth muscle cells [32]. In the present study, the NIH 3T3 mouse embryonic fibroblasts cell line was used to evaluate the bioactivity of both standard and released HB-EGF. After supplementing the cells with HB-EGF at various concentrations, the % relative cell growth with respect to control containing without HB-EGF was monitored by MTT assay and presented in Fig. 6. When compared to control, the cells treated with HB-EGF displayed increased % cell growth and thus revealed that the HB-EGF possesses strong mitogenic potency [33]. However, the maximum cell growth was found at 10 ng/ml HB-EGF and after that a slight decrease in the cell growth was observed. The % cell growth was increased irrespective of the concentration of HB-EGF until 48 h and followed by a decrease in % cell growth for the cells treated with HB-EGF at a concentration of about 0.5–5 ng/ml. The bioactivity of the HB-EGF released from the microspheres in presence of collagenase was evaluated and presented in Fig. 7. The amount of HB-EGF present in the release medium was quantified by ELISA (Human HB-EGF Duoset ELISA kit, R&D systems, Minneapolis, USA) and 10 ng/ml equivalent medium was treated with the cells. The Fig. 7 reveals that there was difference in mitogenic potential of the HB-EGF released from CMS and HMS. When compared with standard, the HMS displayed an identical % cell growth during 48 h and slightly lower % cell growth during 72 h. Whereas CMS displayed significantly lower % cell growth when compared to standard HB-EGF during both 48 and 72 h. The study suggested that the HB-EGF released from HMS was functionally active when compared to HB-EGF released from CMS. This is demonstrated by the presence of heparin in HMS, which binds with HB-EGF and stabilizes in the high protease environment.
Cytotoxicity
The fluoroprobe Calcein AM was used to observe the cell viability under fluorescence microscope. Calcein AM is a membrane permeant, non-fluorescent molecule, once inside the cells it is hydrolysed by endogenous esterases into green fluorescent calcein and retained in the cytoplasm of the live cells (Fig. 8a). When the cells were exposed into the HMS they were migrated towards the microspheres and attached over the surface of the HMS as shown in photograph taken after 48 h (Fig. 8b). Moreover, after 96 h it was observed that the cells near the microspheres were completely entrapped and accumulated over the surface of the HMS as shown in Fig. 8c. This clearly indicates that the developed microspheres were cell friendly and non-toxic. This finding also demonstrated that the HMS possess strong affinity to the fibroblasts and have potential application in tissue engineering as a carrier for cell delivery.
Conclusions
The present study demonstrated that the development of heparin-functionalized gelatin microspheres (HMS) for heparin-binding growth factor delivery systems using EDC and NHS coupling protocol. About 1.97 μmol of heparin per gram of the HMS was conjugated and this demonstrated significant protection against enzymatic degradation. The developed system was also found to be non-toxic and useful for prolonged growth factors release. The HMS demonstrated the sustained release of HB-EGF for more than 10 days even in the presence of collagenase at 20 U/ml and most importantly the biological activity of HB-EGF was maintained during the release period. The overall studies suggest that the HMS could be used as a growth factor’s delivery component in tissue engineering scaffolds, particularly for wound-healing applications.
References
Choi JS, Leong KW, Yoo HS (2008) In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 29:587–596
Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC (1994) Cryopreserved saphenous vein allografts for below-knee lower extremity revascularization. Ann Surg 219:688–692
Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK (1996) Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Investig Dermatol 107:743–748
Falanga V, Eaglstein WH (1993) The “trap” hypothesis of venous ulceration. Lancet 341:1006–1008
Higley HR, Ksander GA, Gerhardt CO, Falanga V (1995) Extravasation of macromolecules and possible trapping of transforming growth factor-β in venous ulceration. Br J Dermatol 132:79–85
Robson MC, Mustoe TA, Hunt TK (1998) The future of recombinant growth factors in wound healing. Am J Surg 176:80S–82S
Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL (2003) Growth factors in the treatment of diabetic foot ulcers. Br J Surg 90:133–146
Smiell JM, Wieman TJ, Steed DL, Perry BH, Sambson AR, Schwab BH (1999) Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 7:335–346
Andreopoulos FM, Persaud I (2006) Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials 27:2468–2476
Zhu XH, Tabata Y, Wang HH, Tong Y (2008) Delivery of basic fibroblast growth factor from gelatin microsphere scaffold for the growth of human umbilical vein endothelial cells. Tissue Eng Part A 14:1939–1947
Gospodarowicz D, Cheng J (1986) Heparin protects basic and acidic FGF from inactivation. J Cell Physiol 128:475–484
Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, Van Aken WG, Feijen J (2001) Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials 22:2291–2299
Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y (2001) Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res 56:216–221
Chung Y, Tae G, Yuk SH (2006) A facile method to prepare heparin-functionalized nanoparticles for controlled release of growth factors. Biomaterials 27:2621–2626
Lee JS, Go DH, Bae JW, Lee SJ, Park KD (2007) Heparin conjugated polymeric micelle for long-term delivery of basic fibroblast growth factor. J Control Release 117:204–209
Tabata Y, Hijikata S, Ikada Y (1994) A novel skeletal drug delivery system using self-setting bioactive glass bone cement. III: the in vitro drug release from bone cement containing indomethacin and its physicochemical properties. J Control Release 31:111–119
Morimoto K, Chono S, Kosai T (2008) Design of cationic microspheres based on aminated gelatin for controlled release of peptide and protein drugs. Drug Deliv 15:113–117
Kosasih A, Bowman BJ, Wigent RJ, Ofner CM (2000) Characterization and in vitro release of methotrexate from gelatin/methotrexate conjugates formed using different preparation variables. Int J Pharm 204:81–89
Wang J, Tabata Y, Morimoto K (2006) Aminated gelatin microspheres as a nasal delivery system for peptide drugs: evaluation of in vitro release and in vivo insulin absorption in rats. J Control Release 113:31–37
Tabata Y, Ikada Y (1998) Protein release from gelatin matrices. Adv Drug Deliv Rev 31:287–301
Adhirajan N, Shanmugasundaram N, Shanmuganathan S, Babu M (2009) Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur JPharm Sci 36:235–245
Radulescu A, Zhang H, Chen CL, Chen Y, Zhou Y, Yu X, Otabor L, Olson JK, Besner GE (2011) Heparin-binding EGF-like growth factor promotes intestinal anastomotic healing. J Surg Res 171:540–550
Adhirajan N, Shanmugasundaram N, Babu M (2007) Gelatin microspheres cross-linked with EDC as a drug delivery system for doxycycline: development and characterization. J Microencapsul 24:659–671
Smith PK, Mallia S, Hermanson GT (1980) Colorimetric method for the assay of heparin content in immobilized heparin preparations. Anal Biochem 109:466–473
Olde D, Dijkstra PJ, Van Luyn VKA, Van Wachem PB, Nieuwenhuis P, Feijen J (1996) Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 17:765–773
Kuijpers AJ, Engbers GHM, Krijgsveld J, Zaat SAJ, Dankert J, Feijen J (2000) Cross-linking and characterisation of gelatin matrices for biomedical applications. J Biomater Sci Polym Ed 11:225–243
van Kuppervelt TM, Veerkamp JH (1994) Application of cationic probes for the ultrastructural localization of proteoglycans in basement membranes. Microsc Res Techn 28:125–140
Nimni ME, Harkness RD (1988) Molecular structure and function of collagen. In: Nimni ME (ed) Collagen, vol I. CRC Press, Boca Raton, pp 1–77
Yao C, Roderfeld M, Rath T, Roeb E, Bernhagen J, Steffens G (2006) The impact of proteinase-induced matrix degradation on the release of VEGF from heparinized collagen matrices. Biomaterials 27:1608–1616
Dogan AK, Gumusderelioglu M, Aksoz E (2005) Controlled release of EGF and bFGF from dextran hydrogels in vitro and in vivo. J Biomed Mater Res B Appl Biomater 74:504–510
Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y (2004) Collagenous matrices as release carriers of exogenous growth factors. Biomaterials 25:4513–4520
Raab G, Klagsbrun M (1997) Heparin-binding EGF-like growth factor. Biochim Biophys Acta 1333:F179–F185
Berner G, Higashiyama S, Klagsbrun M (1990) Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul 1:811–819
Acknowledgments
This work was supported by Department of Biotechnology, Ministry of Science and Technology, New Delhi, India (Grant No. BT/PR8203/MED/14/1237/2006). The authors thank the Director, CLRI, for giving us the opportunity and encouragement to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adhirajan, N., Thanavel, R., Naveen, N. et al. Functionally modified gelatin microspheres as a growth factor’s delivery system: development and characterization. Polym. Bull. 71, 1015–1030 (2014). https://doi.org/10.1007/s00289-014-1108-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1108-3