Abstract
The random copolymers of ε-caprolactone (CL) and 2,2-ethylenedioxy propane-1,3-diol carbonate (EOPDC) were synthesized in bulk at 120 °C using Sn(Oct)2 as a catalyst. The poly(EOPDC-co–CL)s obtained were characterized by FT IR, 1H NMR, 13C NMR, GPC and DSC. The copolymers were obtained with yield of 84.2–97.8 %. The number-average molecular weight of the copolymer is 2.75–7.76 × 104 with a polydispersity of 1.52–1.68. The properties of the copolymer including the enzymatic degradation by Pseudomonas Cepacia lipase and drug-controlled release property were also investigated. The results showed that the copolymers are degradable at physiological conditions, and their degradation rate and release of Tegafur in the copolymers increase with increasing CL content in the copolymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodegradable polymers belong to the class of the most attractive biomaterials because of their wide applications in biomedical fields [1]. Most of the synthetic biodegradable polymers are aliphatic polyesters, among them, ε-polycaprolactone (PCL) is one of the most important biodegradable polymers due to its biodegradability, biocompatibility, non-toxicity and good permeability to drug [2]. However, PCL has slow degradation rate because of its poor hydrophilicity and high crystallinity that limit its applications in the biomedical fields. To satisfy the requirements of industrial, agricultural and specific medical applications, many modification strategies have been chosen to optimize certain properties of the biomaterials. Modification via copolymerization is an effective way to obtain the materials with desirable properties, a lot of copolymers of ε-caprolactone (CL) with other monomers such as lactide (LA) [3] and glycolide [4, 5], 5-methyl-5-benzyloxycarbonyl-1,3-dioxane-2-one (MBC) [6, 7], trimethylene carbonate (TMC) [8–11], 2,2-dimethyl trimethylene carbonate (DTC) [12–14], 1,4-dioxane-2-one [15] and poly(ethylene glycol) (PEG) [16–18] have been extensively investigated to improve their physical or chemical properties and further expand applications of PCL.
Aliphatic polycarbonates represent one family of bioresorbable materials used for biomedical applications because of their good biocompatibility, low toxicity, and biodegradability [19, 20]. Recently, increasing attention has been paid to the synthetic aliphatic polycarbonates bearing functional groups, such as alkyl [21], allyl [22], OH [23, 24], NH2 [25], COOH [26], COOR [27] and ketone carbonyl group [28], because these functional groups can be used to regulate hydrophilicity/hydrophobicity, permeability, bioresorption and mechanical properties to obtain biodegradable polymers with improved properties.
In our pervious studies[29, 30], poly(2,2-ethylenedioxy propane-1,3-diol carbonate) (PEOPDC) is a novel biodegradable aliphatic polycarbonate based on dihydroxyacetone with ethylene ketal-protected carbonyl group, which possess good biocompatibility and biodegradation in comparison with poly(2, 2-dimethyl trimethylene carbonate) (PDTC).
As part of our continuing efforts in the study of novel biodegradable polycarbonates homopolymers or copolymers, in this paper, syntheses and characterization of novel polycaprolactone and aliphatic polycarbonate aliphatic polycarbonate copolymers by random ring-opening copolymerization of CL and 2,2-ethylenedioxy-1,3-propanediol carbonate (EOPDC) was described, and the properties of the copolymer including enzymatic degradation properties and preliminary drug-controlled release property were also investigated.
Experimental
Materials
Toluene was dried over Na before use. Stannous octanoate (95 %) and ε-caprolactone were purchased from Aldrich. Pseudomonas Cepacia lipase (40 U/mg) was purchased from Fluka. The initiator Sn(Oct)2 was purified by distillation under reduced pressure and then dissolved in dry toluene. ε-Caprolactone was dried over CaH2 and purified by distillation under reduced pressure prior to use. 5-Fluoro-1-(tetrahydro-2-furyl)uracil (Tegafur) was recrystallized with ethanol. Magnesium sulfate, chloroform, Tris, NaN3, THF and ethyl acetate (Shanghai Chemical Reagent Co., China) were of analytical grade and used as received. Cyclic monomers, EOPDC, were synthesized as described in literature [29].
Measurements
IR spectra (in KBr pellets) were recorded on Perkin Elmer-2 spectrometer. UV spectra were recorded on Perkin-Elmer Lambda Bio 40 UV/Vis Spectrophotometer. 1H NMR analysis was performed on a Mercury VX-300 spectrometer using CDCl3 as a solvent. 13C NMR spectra were recorded on a Mercury VX-300 spectrometer at 62.5 MHz using CDCl3 as a solvent. The concentration of Tegafur was determined by Shimadzu UV-240. Molecular weight was determined by a gel permeation chromatographic (GPC) system (2690D, Waters) with a 2410 refractive-index detector, and Shodex K803 and K805 columns. Chloroform was used as the eluent at a flow rate of 1.0 ml/min. The glass transition temperatures of polymers were measured by differential scanning calorimeter (DSC) (Perkin-Elmer Pyris-1). Samples were heated from −25 °C to 200 °C at a heating rate of 20 °C/min.
Synthesis of copolymer of EOPDC and CL
Prescribed amounts of EOPDC and CL with Sn(Oct)2 as a catalyst were put into polymerization tubes. The tubes were purged with nitrogen three times, and then sealed under vacuum and placed in an oil bath under certain conditions. After the reaction, the products were dissolved in CH2Cl2, and precipitated with CH3OH. The precipitated poly(EOPDC-co–CL) was dried in vacuum at 50 °C for 24 h.
Enzymatic degradation
The polymer films were prepared by solution-casting method, and the size and weight of the films used were about 10 × 10 mm2 and 10–15 mg, respectively. Enzymatic degradation experiments was performed at 37 °C by immersing copolymer films in 5 ml Tris–HCl buffer solution (0.1 M, pH 8.6) in the presence of 1 mg Pseudomonas Cepacia lipase and 1 mg sodium azide, and the buffer-enzymatic solution was changed every 24 h to maintain the enzymatic activity. These films were taken out from the solution over predetermined time intervals, then washed with distilled water and dried to a constant weight in vacuum. The degradation rate was determined by the weight loss. Weight loss is defined as: weight loss (%) = [(W i − W t )/W i] × 100 %, where W i is initial weight and W t is weight after degradation at different time intervals.
In vitro drug-controlled release
A mixture of 50 mg polymer and 5 mg Tegafur was dissolved in 1 ml CH2Cl2, then the solution was cast onto Teflon board at room temperature, the film was dried to constant weight in vacuum. The sample was immersed in 50 ml phosphate buffer solution (pH 7.4, 0.1 M) at 37 °C, 10 ml solution was taken off for released Tegafur content measurement and the same volume of fresh buffer solution was added at predetermined time intervals. The concentration of Tegafur was determined by UV spectroscopy at maximal absorption wavelength (λ max = 270.8 nm). The rate of controlled drug release was measured by accumulatively released weight of Tegafur according to the calibration curve of Tegafur.
Results and discussion
Synthesis and characterization of the copolymers
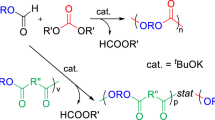
Novel biodegradable copolymers were synthesized by ring-opening polymerization of EOPDC and CL, using Sn(Oct)2 as catalyst in bulk (Scheme 1). By varying the comonomer feed ratio, the copolymerization was carried out at 120 °C for 24 h when the [monomer]/[catalyst] was 1,000. The effects of different molar feed ratios of EOPDC/CL on yield and number-average molecular weight (Mn) of the copolymers were investigated. The results of the copolymerization are shown in Table 1. It can be seen that the molar ratio of EOPDC in the corresponding copolymers is almost the same as that in feeds, which indicates that EOPDC has the same polymerization reactivity as CL in the copolymerization, but the influence of the feed molar ratio of EOPDC/CL on Mn and yield of the copolymers obtained was not obvious. GPC results show that all of the polymers obtained have unimodal molecular weight distributions, so it can be preliminarily concluded that the two monomers of EOPDC and CL have copolymerized.
The copolymers were characterized by FTIR, 1H NMR and 13C NMR. In the FTIR spectra, there were four ester carbonyl absorption peaks at about 1,750 cm−1. The characteristic bands around 1,755, 1,748 cm−1 was due to polycarbonate ester C=O stretch, whereas 1,739, 1,733 cm−1 was due to polycaprolactone ester C=O stretch, and the characteristic absorption band at 1,253, 1,189, 1,043 cm−1 was due to the C–O–C stretching vibration in copolymers, which might indicate the formation of random copolymers. The 1H NMR spectrum of the copolymer with the molar feed ratio of EOPDC/CL (50/50) is presented in Fig. 1. As the EOPDC unit was symmetric, the chemical shifts of two methylene protons in 5-membered ring (OCH 2CH 2O) (aH) were at the same site δ = 4.01 ppm, and the chemical shifts of other two methylene protons link to carbonate ester groups (CH 2OCOCH 2) (bH) were at the same site δ = 4.18 ppm [29]. The chemical shift at 4.06 ppm belonged to the characteristic peaks of (COCH2CH2CH2CH2CH 2O)(cH) of CL units, 2.31 ppm to (COCH 2CH2CH2CH2CH2O) (gH), and 1.39 ppm to (COCH2CH2CH 2CH2CH2O) (eH) of CL units, and 1.66 ppm to (COCH2CH 2CH2CH 2CH2O) (dH and fH)of CL units. 1H NMR was also used to measure the copolymer compositions by comparing the relative areas of peaks corresponding to the protons of the EOPDC and CL repeat units. It can be found that the molar ratio of EOPDC in the corresponding copolymers is hardly the same as that in feeds. The 13C NMR spectrum of the copolymer with the molar feed ratio of 50/50 EOPDC/CL is shown in Fig. 2. Signals from the EOPDC units and the CL units can be clearly observed. The detailed assignments are shown in Fig. 2. It can be seen, comparing with corresponding carbon resonance of homopolymer, the carbonyl carbon resonance of the EOPDC in the copolymer splits into two peaks at about 154 ppm (dC), and the carbonyl carbon resonance of CL units was two peaks at about 173 ppm (jC), whereas carbon resonance of bC and eC also splits into two peaks effected by copolymerization, which is due to the different chemical environments caused by the different sequences in the copolymer chain. Therefore, from the 13C NMR spectra, we can further confirm that the two comonomers are copolymerized by random copolymerization.
Thermal properties of copolymers
The DSC curves of poly(EOPDC-co–CL) were measured and the results are shown in Fig. 3. It can be seen that a endothermic peaks at 62 °C which corresponded to the melting of the PCL, but melting temperature of the copolymer decrease with increasing EOPDC content. When the molar ratio of in feed is more than 50 %, the melting peak is not observed. It is probably because that the PCL regular structure is disturbed, and its crystallization is retarded by introducing the amorphous PEOPDC units into the PCL main chain.
Enzymatic degradation of copolymers
The enzymatic degradation properties of poly(EOPDC-co–CL) copolymers were evaluated by the weight loss at different degradation time, the results are presented in Fig. 4. It can be seen from Fig. 4 that the degradation rate increases greatly with increasing the molar content of PCL. Thus, the copolymer degradation properties can be adjusted by varying the comonomer feed ratio to adapt to the different applications.
In vitro-controlled drug release of copolymers
Anti-tumor drug Tegafur was selected as a model drug to investigate the in vitro-controlled drug release property of the poly(EOPDC-co–CL) copolymers. The release rate was monitored by determining the concentration of accumulatively released Tegafur. The preliminary results are shown in Fig. 5. It can be seen that the rate of controlled drug release from three copolymers was very slow because mainly these copolymers had slow degradation rate in PBS, and that the initial burst release was obvious when CL content reach 90 %. Whereas rate of drug release maintain very slow increasing when CL content reach 50 %. It demonstrates that the behavior of release drug can be controlled by varying the content of EOPDC incorporated into the CL main chain.
Conclusions
Novel biodegradable copolymers of EOPDC and CL with high molecular weight were synthesized under different conditions by ring-opening polymerization. The results show that EOPDC has almost the same polymerization reactivity as CL. The thermal properties can be adjusted by varying the composition of the copolymers. The enzymatic degradation rate increases greatly with increasing the molar content of PCL. The behavior of release drug can be controlled by varying the content of EOPDC incorporated into the CL main chain.
References
Martina M, Hutmacher DW (2007) Biodegradable polymers applied in tissue engineering research: a review. Polym Int 56:145–157
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484–3504
Wei ZY, Liu L, Yu FY, Wang P, Qu C, Qi M (2008) Synthesis of poly(epsilon-caprolactone)-poly(l-lactide) block copolymers by melt or solution sequential copolymerization using nontoxic dibutylmagnesium as initiator. Polym Bull 61:407–413
Sivalingam G, Madras G (2004) Thermal degradation of binary physical mixtures and copolymers of poly(ε-caprolactone), poly(d, l-lactide), poly(glycolide). Polym Degrad Stab 84:393–398
Cho DK, Park JW, Kim SH, Kim YH, Im SS (2003) Effect of molecular orientation on biodegradability of poly(glycolide-co-epsilon-caprolactone). Polym Degrad Stab 80:223–232
Storey RF, Mullen BD, Melchert KM (2001) Synthesis of novel hydrophilic poly(ester-carbonates) containing pendent carboxylic acid groups. J Macromol Sci, Pure Appl Chem 38:897–917
Guan HL, Xie ZG, Tang ZH, Xu XY, Chen XS, Jing XB (2005) Preparation of block copolymer of epsilon-caprolactone and 2-methyl-2-carboxyl-propylene carbonate. Polymer 46:2817–2824
Pego AP, Luyn MJAV, Brouwer LA, Wachem PBV, Poot AA, GrijiPma DW (2003) In vivo behavior of poly(1,3-trimethylene carbonate) and copolymers of 1,3-trimethylene carbonate with d, l-lactide or epsilon-caprolactone: degradation and tissue response. J Biomed Mater Res 67(A):1044–1054
Ling J, Zhu WP, Shen ZQ (2004) Controlling ring-opening copolymerization of epsilon- caprolactone with trimethylene carbonate by scandium tris(2,6-di-tert -butyl-4-methylphenolate). Macromolecules 37:758–763
Schappacher M, Fabre T, Mingotaud AF, Soum A (2001) Study of a (trimethylenecarbonate-co-epsilon-caprolactone) polymer—part 1: preparation of a new nerve guide through controlled random copolymerization using rare earth catalysts. Biomaterials 22:2849–2855
Fabre T, Schappacher M, Bareille R, Dupuy B, Soum A, Bertrand-Barat J, Baquey C (2001) Study of a (trimethylenecarbonate-co- epsilon-caprolactone) polymer—part 2: in vitro cytocompatibility analysis and in vivo ED1 cell response of a new nerve guide. Biomaterials 22:2951–2958
Zhu GX, Ling J, Shen ZQ (2003) Isothermal crystallization of random copolymers of epsilon-caprolactone with 2,2-dimethyltrimethylene carbonate. Polymer 44:5827–5832
Shen ZQ, Zhu GX, Ling J (2002) Homo- and copolymerization of epsilon-caprolactone and 2,2-dimethyltrimethylene carbonate by rare earth initiators. Chin J Chem 20:1369–1374
Yasuda H, Aludin MS, Kitamura N, Tanabe M, Sirahama H (1999) Syntheses and physical properties of novel optically active poly(ester-carbonate)s by copolymerization of substituted trimethylene carbonate with epsilon-caprolactone and their biodegradation behavior. Macromolecules 32:6047–6057
Raquez JM, Degee P, Narayan R, Dubois P (2004) Diblock copolymers based on 1,4-dioxan-2-one and epsilon-caprolactone: characterization and thermal properties. Macromol Chem Phys 205:1764–1773
Ge H, Hu Y, Jiang X, Cheng D, Yuan Y, Bi H, Yang C (2002) Preparation, characterization, and drug release behaviors of drug nimodipine-loaded poly(epsilon-caprolactone)-poly(ethylene oxide)-poly(epsilon-caprolactone) amphiphilic triblock copolymer micelles. J Pharm Sci 91:1463–1473
He F, Li S, Vert M, Zhuo R (2003) Enzyme-catalyzed polymerization and degradation of copolymers prepared from ε-caprolactone and poly(ethylene glycol). Polymer 44:5145–5151
You JH, Choi SW, Kim JH (2008) Synthesis and microphase separation of biodegradable poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) multiblock copolymer films. Macromol Res 16:609–613
Rokicki G (2000) Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog Polym Sci 25:259–342
Feng J, Zhuo RX, Zhang XZ (2012) Construction of functional aliphatic polycarbonates for biomedical applications. Prog Polym Sci 37:211–236
Mullen BD, Tang CN, Storey RF (2003) New aliphatic poly(ester-carbonates) based on 5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one. J Polym Sci, Part A: Polym Chem 41:1978–1991
He F, Wang YP, Liu G, Jia HL, Feng J, Zhuo RX (2008) Synthesis, characterization and ring-opening polymerization of a novel six-membered cyclic carbonate bearing pendent allyl ether group. Polymer 49:1185–1190
Wang XL, Zhuo RX, Liu LJ, He F, Liu G (2002) Synthesis and characterization of novel aliphatic polycarbonates. J Polym Sci, Part A: Polym Chem 40:70–75
Li ZM, Yan GP, Ai CW, Zhang Q, Li L, Liu F, Yu XH, Zhao BA (2012) Synthesis and properties of polycarbonate copolymers of trimethylene carbonate and 2-phenyl-5,5- bis(hydroxymethyl) trimethylene carbonate. J Appl Polym Sci 124:3704–3713
Sanda F, Kamatani J, Endo T (2001) Synthesis and anionic ring-opening polymerization behavior of amino acid-derived cyclic carbonates. Macromolecules 34:1564–1569
Al-Azemi TF, Bisht KS (2002) One-step synthesis of polycarbonates bearing pendant carboxyl groups by lipase-catalyzed ring-opening polymerization. J Polym Sci, Part A: Polym Chem 40:1267–1274
Zhou Y, Zhuo RX, Liu ZL (2005) Synthesis and characterization of novel aliphatic poly(carbonate-ester)s with functional pendent groups. Macromol Rapid Commun 26:1309–1404
Zelikin AN, Zawaneh PN, Putnam D (2006) A functionalizable biomaterial based on dihydroxyacetone. an intermediate of glucose metabolism. Biomacromolecules 7:3239–3244
Wang LS, Cheng SX, Zhuo RX (2004) Novel biodegradable aliphatic polycarbonate based on ketal protected dihydroxyacetone. Macromol Rapid Commun 25:959–963
Wang LS, Jiang XS, Wang H, Cheng SX, Zhuo RX (2005) Preparation and cytotoxicity of novel aliphatic polycarbonate synthesized from dihydroxyacetone. Chin Chem Lett 16:572–574
Acknowledgments
The authors are grateful for the financial support of national key basic research and development program (G1999064703) and natural science foundation of China (Grant No. 20174029).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Ls., Cheng, Sx. & Zhuo, Rx. Syntheses and properties of novel copolymers of polycaprolactone and aliphatic polycarbonate based on ketal-protected dihydroxyacetone. Polym. Bull. 71, 47–56 (2014). https://doi.org/10.1007/s00289-013-1044-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-1044-7