Abstract
A suitable Ziegler–Natta catalyst was prepared by supporting TiCl4 on sodium montmorillonite (Na+MMT) modified by butyl octyl magnesium (BOM). This catalyst was applied for the polymerization of ethylene toward a polyethylene (PE)/Na+MMT nanocomposite. Catalyst behavior and nanocomposite properties were studied. It was found that catalyst activity was acceptably high. In addition, it had a smooth rate during ethylene polymerization. Transmission electron microscopy image and X-ray diffraction pattern evidenced an excellent exfoliation of the Na+MMT layers in the polymer matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyolefins are commercial materials having the largest tonnage in the polymeric industries because they enjoy abundant monomers and valuable properties leading to extensive applications in various industries. In addition, polyolefins are often filled with organic or inorganic components to enhance their physical and mechanical properties [1–3].
Over the past two decades, there has been a growing remarkable academic and industrial interest in developing polymer nanocomposites because of their noticeably enhanced properties compared to virgin polymer or conventional micro- or macro-composites. In addition, reinforced polyolefins can be one of the desired choices for replacing high-performance engineering plastics [4–6].
PE/MMT nanocomposites have greatly progressed due to their potential as alternative low-cost and high-performance materials. This potential is derived from the high aspect ratio of the MMT when its structure is delaminated by the polymer. In fact, the delamination of the MMT layers results in synergistic effect of the nanoscale structure which maximizes the interactions between MMT and polymer molecules [7–9]. As expected, such products show high mechanical properties which are desired for many applications [9, 10].
According to literature, the main approaches for producing nanocomposites are solution, melting and in situ polymerization. Among them, the latest is more attractive because the dispersion obtained by in situ polymerization was the most efficient, in particular, in fully exfoliated polyolefin/MMT nanocomposite formation [6, 11–14].
Metallocene and Ziegler–Natta catalysts supported on MMT have been mostly employed for the in situ polymerization of ethylene. Metallocene catalysts need an excessive amount of cocatalyst for activation, which is the most critical limitation for their use [15]. Ziegler–Natta catalysts supported on MMT suffer from some deficiencies including low activity, which is the basic restriction for the production of PE/MMT nanocomposites by in situ polymerization, especially from the industrial perspective. In particular, Na+MMT provided the most inefficient support compared to the modified ones. In addition, many of the modified Na+MMT did not show acceptable performance. Furthermore, it was reported that catalyst–MMT intercalation was mostly through physical absorption. These intercalated catalysts were easily removed from inside the interlayer gallery, which led to low intercalative selectivity in the in situ polymerization. Consequently, nanoscopic exfoliation of MMT during the in situ polymerization was hardly complete [16–18].
In this study, our main aim was to produce an exfoliated structure of the PE/Na+MMT nanocomposite by using a Ziegler–Natta type of catalyst supported on Na+MMT with suitable activity, which is the most required situation in clay-based catalysts. For this reason, we modified Na+MMT using BOM, which was a new approach for the modification of Na+MMT. Then, its performance was investigated in the ethylene polymerization for producing PE/Na+MMT nanocomposite. This novel approach provides an unprecedented opportunity of producing highly exfoliated PE/Na+MMT nanocomposites via in situ polymerization.
Experimental
Materials
Ethylene (polymer grade), nitrogen (>99.99 %) and heptane (H2O < 3 ppm) were purchased from Linde (Germany), Arkan Gas Co. (Iran) and Pentane Chemical Industries (Iran). TiCl4 and triethyl aluminum (TEA) were purchased from Fluka Co. (Switzerland). Chloroform and ethanol (extra pure grade) were purchased from Merck Co. (Germany). Butyl octyl magnesium (BOM) and Na+MMT were prepared from Chemtura (Germany) and Southern Clay (USA), respectively.
Modification of Na+MMT
Na+MMT was heated at 80 °C in vacuo for 4 h. 10 g of the above Na+MMT was added to a 500-mL flat-bottomed flask containing 250 mL of heptane under N2 and vigorously mixed for 10 min. Then, 2.7 g of BOM was added to the flask during 15 min. The mixture was warmed up to 60 °C and further mixed for 10 h. The product was washed eight times and used as the support for catalyst preparation.
Preparation of catalyst
The preparation of the catalyst was carried out in a 1.0-L steel jacket Buchi autoclave reactor equipped with a mechanical seal stirrer at a speed of 300 rpm. After running out of moisture and oxygen by nitrogen, 350 mL of heptane containing 10 g of the modified Na+MMT was added and then mixed for 10 min. The temperature was increased to 70 °C, and 1.5 mL of chloroform in 20 mL of heptane was added dropwise to the mixture during 90 min and further mixed at 75–78 °C for 2 h. The temperature was increased to 85 °C and 0.1 mL of ethanol in 10 mL of heptane was injected and mixed for 1 h. TiCl4 (2 mL) in 10 mL of heptane was introduced to the reactor over 25 min and further mixed at 94 °C for 2 h. Finally, the produced catalyst was washed eight times with heptane until no traces of titanium were detected in the washing liquid.
Polymerization
Polymerization was carried out in a 1.0-L steel jacket Buchi autoclave reactor equipped with a mechanical seal stirrer in the slurry phase.
After running out of moisture and oxygen by nitrogen, 500 mL of heptane was added and mixed for 10 min under nitrogen. 2 mL of TEA (1 M in heptane) was added as a cocatalyst to the reactor and then the reactor was heated to 70 °C. After the injection of 400 mg of the catalyst, ethylene was supplied continuously at a pressure of 8 bars during 2 h. The ethylene consumption was measured by using a mass flow meter (Brooks, Holland). After polymerization, the untreated gases were slowly released and the polymer was then filtered and dried.
Characterization
The titanium content of the synthesized catalyst was measured by UV–visible method (at a wavelength of 410 nm) on Shimadzu, UV-1650 PC (Japan) [19]. The catalyst contained 1.2 wt% of Ti.
X-ray diffraction (XRD) was used for considering the delamination in the silicate layers of clay under ethylene polymerization. The experiments were carried out by the Philips XRD Instrument (X’Pert MPD, Holland) with Co kα radiation (λ = 1.78897 Å) at room temperature.
The degree of crystallinity (X c), heat of fusion (ΔH m), melting and crystallization points (T m and T c) of the nanocomposite were studied by differential scanning calorimetry (DSC) [20]. The measurements were performed by a Metler Toledo 822e calorimeter at a heating or cooling rate of 10 °C/min under nitrogen in the range of 25–200 °C. The Na+MMT content of the nanocomposite was determined by thermal gravimetric analysis (TGA) at a heating rate of 20 °C/min under nitrogen in a scanning range of 35 to about 750 °C (Universal V4.1D TA Instruments). The density of the nanocomposite was measured according to ASTM D 1505.
The molecular weight and molecular weight distribution of the produced polymer were measured by the gel permeation chromatography (GPC) method by Waters Instrument, model 150-C. The operating conditions were set according to [21].
The nanometer structure of the nanocomposite was investigated by TEM (Philips CM30) at an acceleration voltage of 250 kV. For the TEM analysis, the sample was melted and then cooled to room temperature. It was cut with an ultramicrotome diamond knife at an angle of 35° (Leica EM UC7, Germany). The cut thickness was about 40 nm. Scanning electron microscopy (SEM) was used for studying the morphology of the polymer powder (Cam Scan MV 2300).
Results and discussion
Catalyst and polymerization
The Na+MMT/BOM compound produced by the treatment of Na+MMT with BOM was used as a support in catalyst preparation. Table 1 shows the BET surface area of the Na+MMT, modified Na+MMT by BOM and the produced catalyst. With regard to the table, the surface area of Na+MMT improved under treatment with BOM and also with catalyst preparation, increasing from about 10 m2/g in unmodified Na+MMT to about 23 m2/g in Na+MMT/BOM and catalyst. In contrast, a slight reduction in the average pore diameter was observed. Overall, an increase in the surface area can lead to improvement in the behavior of the Ziegler–Natta type of catalysts in olefin polymerization [2].
The prepared Na+MMT/BOM/chloroform/EtOH/TiCl4/TEA catalyst system showed an activity of 340 kgPE/molTi in ethylene polymerization. Although the produced catalyst displayed low activity compared to the modern and commercial Ziegler–Natta catalysts, its activity was suitable for the production of the PE/Na+MMT nanocomposites having reasonable Na+MMT contents. The main reason for the deficiency of the non-modified Na+MMT-supported catalyst can be contributed to the presence of the hydroxyl group on Na+MMT. The other reason is the low surface area of the clay-supported catalysts compared to that of the conventional Ziegler–Natta catalysts [18, 22].
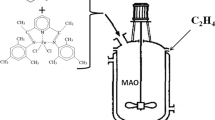
As mentioned, the non-modified Na+MMT-supported catalyst showed very low activity in olefin polymerization. The higher performance of the produced Na+MMT/BOM-supported catalyst in the ethylene polymerization can be contributed to the role of BOM. In fact, BOM can act as both an efficient surface modifier for Na+MMT and the second support in catalyst preparation. In these cases, BOM offers the most loading sites and, consequently, the titanium catalyst is avoided from directly anchoring on the clay surface [23]. The scheme of the possible reactions has been shown in Fig. 1.
It was proven that the Ti species in such catalyst systems was mainly supported on the MgCl2, probably through a Cl bridge, instead of the layer surface of MMT. In other words, MgCl2 may form a single molecular layer along the inner surfaces of MMT, avoiding the formation of non/low active species (Si–O–Ti), resulting from the reaction of OH on the MMT layer surface with TiCl4 [24, 25].
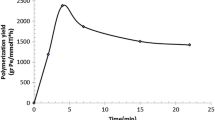
Figure 2 illustrates the rate of ethylene polymerization using the above Na+MMT-supported catalyst system. According to the figure, this catalyst displayed a smooth reduction in the rate of ethylene consumption through polymerization, decreasing from around 20 g/h at the beginning of polymerization to about 10 g/h after 2 h of polymerization.
PE/Na+MMT nanocomposite characterization
Figure 3 presents the XRD patterns of Na+MMT, Na+MMT-BOM, catalyst and nanocomposite. According to the figure, scattering angle 2θ values for Na+MMT, Na+MMT-BOM and catalyst were 7.72, 8.43 and 9.03°, corresponding to a basal spacing of 11.44, 10.47 and 9.77 Å, respectively. This indicates that the stacking order for Na+MMT shifted after the treatment of Na+MMT with BOM and also catalyst supporting. Such changes implied a slight collapse of the Na+MMT layers, which can be explained by the loss of hydrating water from the clay surface via reaction with BOM and also by changes in the nature of the interlayer cations and their hydration behavior [18].
On the contrary, the XRD pattern of the nanocomposite showed no diffraction peak. This result indicates that the produced PE intercalated into the Na+MMT layers and destroyed the clay structure toward the exfoliation of the Na+MMT layers.
The TEM images are shown in Fig. 4. These images confirmed the exfoliated Na+MMT in the PE matrix. Furthermore, the TEM images illustrated a fairly uniform distribution of the Na+MMT layers. This indicates that ethylene polymerization predominantly occurred inside the galleries of the silicate layers of Na+MMT and contributed to the delamination of the clay layers during the polymerization. Consequently, the exfoliated structure of the silicate layers was formed.
Physical properties
The values of T m, ΔH m, T c and X c of the produced nanocomposite are listed in Table 2. According to the table, T m and T c of the nanocomposite were about 136 and 119 °C, which were close to those of the pure PE. In addition, the degree of crystallinity of the nanocomposite was around 55 %, which was slightly lower than that of pure PE. The reason is that the clay decreases the number of crystalline PE chains through preventing the cell growth mechanism during crystallization [10].
The density of the prepared nanocomposite was about 0.9529 g/cm3. It is clear that the density of the produced nanocomposite was slightly less than that of the pure PE in spite of using no comonomer in the polymerization. This can be contributed to the decrease in the crystallinity of the produced nanocomposite compared to the neat polyethylene [10].
Table 3 shows the GPC analysis of the resulting nanocomposite. According to the table, M w, M n, M p and M z of the nanocomposite were about 7.1 × 105, 2.1 × 105, 4.4 × 105 and 29.5 × 105 g/mol, respectively. This means that the introduced catalyst system could produce a high molecular weight nanocomposite. The PDI of the nanocomposite was 3.4, which was slightly lower than that of polyethylene produced by the conventional Ziegler–Natta catalysts [1, 2].
TGA result showed that the PE/Na+MMT nanocomposite lost about 96 % of its weight. This indicates that the nanocomposite contained around 4 wt% of Na+MMT. The thermal decomposition temperature of the produced nanocomposite was about 481 °C which was reasonably more than that of the pure PE (415 °C), as expected from the clay-based nanocomposites. The improvement of the thermal stability of the PE/clay nanocomposite is contributed to the barrier effect of the clay in the nanocomposite.
Morphological studies
Figure 5 shows the SEM images of the Na+MMT, catalyst and PE/Na+MMT nanocomposite. According to the figure, the sample did not have a regular morphology. It has been proven that the polymer tends to replicate the shape of the catalyst particle on which it is produced. In other words, the catalyst particle acts as a template for the growth of the polymer particle [26, 27]. Since the prepared catalyst had an irregular shape, the produced polymer also showed similar morphology. Overall, the literature survey displayed that the nanocomposites produced by using the clay-supported catalysts did not show good morphology (Fig. 5).
Conclusions
BOM acted as a suitable modifier for Na+MMT, so that the produced Na+MMT/BOM-supported Ziegler–Natta catalyst showed high performance in ethylene polymerization toward the PE/Na+MMT nanocomposite. The catalyst polymerized ethylene at lower activity than the modern and conventional fourth-generation Ziegler–Natta catalysts. The XRD and TEM analyses indicated the occurrence of exfoliation. DSC results showed that the melting and crystallization temperatures of the nanocomposite were almost the same as those of pure PE, whereas there was a decrease in the crystallinity degree. However, the thermal stability of the nanocomposite was higher than that of virgin PE.
References
Peacock J (2000) Handbook of polyethylene: structures, properties and applications, 1st edn. Marcel Dekker, New York
Moore EP (1996) Polypropylene handbook, 1st edn. Munich, Hanser
Sedlacek B (1986) Polymer composites, 1st edn. Walter de Gruyter, Berlin
Kocsis JK, Fakirov S (2009) Nano and micro mechanics of polymer blends and composites, 1st edn. Hanser, Monich
Pinnavia TJ, Beale GW (2000) Polymer–clay nanocomposites, 1st edn. Wiley, NewYork
Ray SS, Okamoto M (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci 28:1539–1641
Marques MF, Oliveira MC (2010) Propylene nanocomposites using metallocene catalysts supported on commercial organophilic clays. Polym Bull 64:221–231
Rong J, Jing Z, Li H, Sheng M (2001) A polyethylene nanocomposite prepared via in situ polymerization. Macromol Rapid Commun 22:329–334
Heinemann J, Reichert P, Thomann R, Mulhaupt R (1999) Polyolefin nanocomposites formed by melt compounding and transition metal catalyzed ethene homo- and copolymerization in the presence of layered silicates. Macromol Rapid Commun 20:423–430
Abedi S, Abdouss M, Nekoomanesh-Haghighi M, Sharifi-Sanjani N (2013) PE/clay nanocomposites produced via in situ polymerization by highly active clay-supported Ziegler–Natta catalyst. Polym Bull 70:1313–1325
Alexandre M, Dubois P (2000) Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mat Sci Eng 28:1–63
Baniasadi H, Ramazani A, Javannikkhah S (2010) Investigation of in situ prepared polypropylene/clay nanocomposites properties and comparing to melt blending method. Mat Des 31:76–84
Jin YH, Park HJ, Im SS, Kwak SY, Kwak S (2002) Polyethylene/clay nanocomposite by in situ exfoliation of montmorillonite during Ziegler–Natta polymerization of ethylene. Macromol Rapid Commun 23:135–140
Mittal V (2012) In-situ synthesis of polymer nanocomposites. Wiley-VCH Verlag & Co. KgaA, Weinheim
Yang K, Huang Y, Dong JY (2007) Efficient preparation of isotactic polypropylene/montmorillonite nanocomposites by in situ polymerization technique via a combined use of functional surfactant and metallocene catalysis. Polymer 48:6254–6261
Oliveira M, Marques MF (2011) Polypropylene/organophilic clay nanocomposites using metallocene catalysts through in situ polymerization. Chem Chem Tech 5:201–207
Huang Y, Yang K, Dong JY (2006) Copolymerization of ethylene and 10 undecen-1 ol using a montmorillonite-intercalated metallocene catalyst: synthesis of polyethylene/montmorillonite nanocomposites with enhanced structural stability. Macromol Rapid Commun 27:1278–1283
Maneshi A, Soares JBP, Simon LC (2011) Polyethylene/clay nanocomposites made with metallocenes supported on different organoclays. Macromol Chem Phys 212:216–228
Du K, He HA, Liu X, Han CC (2007) High-performance exfoliated poly(propylene)/clay nanocomposites by in situ polymerization with a novel Z-N/clay compound catalyst. Macromol Rapid Commun 28:2294–2299
Inoue MJ (1963) Studies on crystallization of high polymers by differential thermal analysis. J Polym Sci Part A Gen Papers 1:2697–2709
Abedi S, Hosseinzadeh M, Kazemzadeh MA, Daftari-Besheli M (2006) Effect of polymerization time on the molecular weight and molecular weight distribution of polypropylene. J Appl Polym Sci 100:368–371
Ramazani A, Tavakolzadeh F (2008) Preparation of polyethylene/layered silicate nanocomposites using in situ polymerization approach. Macromol Symp 274:65–71
Cui A, Woo SI (2008) Preparation and characterization of polyethylene (PE)/clay nanocomposites by in situ polymerization with vanadium-based intercalation catalyst. Polym Bull 61:453–460
Zhao HC, Zhang XQ, Yang F, Chen B, Jin YT, Li G, Feng ZL, Huang BT (2003) Synthesis and characterization of polypropylene/momtmorillonite nanocomposites via an in situ polymerization approach. Chinese J Polym Sci 4:413–418
Yang F, Zhang X, Zhao H, Chen B, Huang B, Feng Z (2003) Preparation and properties of polyethylene/montmorillonite nanocomposites by in situ polymerization. J Appl Polym Sci 89:3680–3684
Cecchin G, Marchetti E, Baruzzi G (2001) On the mechanism of polypropene growth over MgCl2/TiCl4 catalyst systems. Macromol Chem Phys 202:1987–1994
Hutchinson RA, Chen CM, Ray WH (1992) Polymerization of olefins through heterogeneous catalysis-X modeling of particle growth and morphology. J Appl Polym Sci 44:1389
Acknowledgments
The authors wish to express their gratitude to the Petrochemical Research and Technology Co. of NPC, Iran National Science Foundation (INSF) and AmirKabir University of Technology for their support in carrying out this project. We would also like to thank Z. Hasanvand, D. Sodbar, S. Mohammadi, N. Zare, L. Baharmand, M. R. Seddigh-e-rad, S. M. Beheshti and B. Raeisi for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abedi, S., Abdouss, M., Daftari-Besheli, M. et al. Highly exfoliated PE/Na+MMT nanocomposite produced via in situ polymerization by a catalyst supported on a novel modified Na+MMT. Polym. Bull. 70, 2783–2792 (2013). https://doi.org/10.1007/s00289-013-0987-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-0987-z