Abstract
In this study, photocurable fluorine-containing coatings were prepared via thiol-ene click chemistry and an in situ sol–gel method. MPTMS was used as a coupling agent to perform both the thiol-ene click reaction and the sol–gel reactions. PFOTES was utilized for the preparation of fluorine-containing coatings. The addition of fluorine and silica showed a significant impact on the properties of the coatings. The addition of silica greatly enhanced the mechanical properties of the coatings. As the fluorine and silica contents were increased in the formulations, flame retardancy, hydrophobicity, and oleophobicity of the coatings increased. High char yields were obtained for the silica- and fluorine-containing samples. Furthermore, the effect of Al2O3 nanoparticles on the properties of the hybrid coatings was investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photopolymerization is a rapid, inexpensive, and simple technique which offers temporal and spatial control of formation of microstructures and allows for processing at ambient conditions. It is widely used in several applications such as inks, coatings, optical devices, electrical and electronic systems, adhesives, dental restoration and also enables the utilization of photolithography, facilitating the synthesis of complex, three-dimensional structures [1, 2]. It is considered as a “green” technology since, in many applications; it reduces the use of volatile organic compounds (VOC) and leads to energy, space, and financial savings [3].
To improve the properties of photocurable coatings, several modifications have been made. One of the most widely used technique to develop UV-curable coatings with desired properties such as high abrasion resistance, high thermal stability, and hardness is to apply sol–gel chemistry which allows preparing organic–inorganic hybrid polymeric networks. These organic–inorganic hybrid materials produced by sol–gel methods are of great interest because they can exploit properties of both the organic (flexibility, toughness) and the inorganic (surface hardness, transparency) components [4, 5]. Generally, for the preparation of UV-curable organic–inorganic hybrid systems via sol–gel chemistry, a two component system is applied where one component consists of acrylic precursors and the other component contains sol–gel precursors such as tetraethylorthosilicate (TEOS), vinyltrimethoxysilane (VTMS), 3-mercaptopropyltrimethoxysilane (MPTMS), methacryloxypropyltrimethoxysilane (MAPTMS), 3-aminopropyltrimethoxysilane (AMPTMS), and glycidoxypropyltrimethoxysilane (GOPS). Also it is very common to hydrolyze sol–gel precursors before mixing with the acrylic components. One of the key points that helps to prevent phase separation between the organic and the inorganic components is to use compounds which possess functional groups that react with both the phases. Thus, VTMS, MAPTMS, or MPTMS are usually used to enhance the compatibility between the organic and inorganic phases. Among these alkoxysilane compounds, MPTMS which contains an –SH bond can add to double bonds via a click type of reaction.
The term “click reaction” was first introduced by Sharpless and coworkers in 2001 and it was defined as a class of selective chemical reactions with high yields, tolerant to various solvents, functional groups and air [6, 7]. The addition reaction between a thiol and an ene is considered as a type of click reaction which has recently been reviewed [8]. Although thiol-ene reaction was first suggested by Posner in 1905 [9] and thiol-ene polymerization was put forward by Kharasch et al. in 1938 [10], the unique features of this useful reaction/polymerization began to interest both the academic and the industrial community in the last decades. Recently, thiol-ene click reaction which is also called as thiol-ene coupling has gained a lot of interest due to its ease of application and desirable properties for a click type of reaction [11, 12]. Thiol-ene click reaction tolerates many functional groups and the reactions take place in mild conditions. Thiol-ene click reactions can be conducted in the absence of solvent and catalyst and the products can be easily purified in high yields [13]. This reaction is also an efficient tool for the functionalization of several surfaces, molecules, and polymers [14–16]. Generally, thiyl radicals are generated under UV irradiation and these radicals add to double bonds (enes) to give anti-Markownikoff products in high yields [17].
In literature, there are several reports in which MPTMS is used for the preparation of hybrid coatings. Hong et al. investigated the effect of component ratios on the properties of UV-curable hybrid coatings where a two component system was used. First, TEOS and MPTMS were mixed in a solvent mixture, then TEOS was partially hydrolyzed with HCL. Hybrid precursors were prepared by mixing this sol–gel mixture at different ratios with a commercial urethane acrylate, TMPTA, and hexanediol diacrylate. Polycarbonate substrates were coated with these precursors. They found that the phase separation was decreased as the amount of MPTMS was increased. Also as the inorganic contents increased, stronger abrasion resistance was observed [18]. A similar study was also conducted by Kim. He also applied a two component system and UV-curable coatings were prepared after the hydrolysis of the sol–gel mixture [19].
In an other study, Li et al. investigated the effects of preparation method on the properties of UV-curable hybrid coatings. UV-curable nanocomposites were prepared via two methods: blending and in situ techniques. In the blending method, acrylic monomers were mixed with the alkoxysilane compounds after hydrolysis. In the other method, hydrolysis of TEOS was performed in situ by mixing TEOS, solvents, ammonia, and the acrylic component, all in one place. For the in situ method, nanosilica particles were not observed due to the limited hydrolysis of silica precursors in the presence of acrylic component [20].
Al2O3 nano- and microparticles have been incorporated into UV-curable coatings due to their outstanding scratch and abrasion resistance [21–24]. Moreover, it was used for the preparation of hydrophobic surfaces [25].
In this study, we prepared fluorine-containing photocurable hybrid coatings via sol–gel technique. But instead of a two component system which involves the hydrolysis of the alkoxysilane precursors, all components were mixed without the hydrolysis of the alkoxysilane groups. The organic phase was modified with MPTMS via thiol-ene click reaction and perfluorooctyltriethoxysilane (PFOTES) was used to introduce fluorine. The organic phase consisted of trimethylolpropane triacrylate (TMPTA). Mechanical properties of the hybrid coatings were determined by stress–strain analysis. The surface topology of the films was observed by a scanning electron microscope (SEM). Hydrophobicity and the oleophobicity of the coatings were determined by contact angle measurements. Thermal and flame retardant properties of the films were evaluated by thermogravimetric analysis (TGA) and limiting oxygen index (LOI) tests, respectively. Also the effect of Al2O3 nanoparticles on the properties of the hybrid coatings was investigated.
Experimental
Materials
MPTMS and PFOTES were purchased from Aldrich and were used as received. TMPTA was obtained from Clariant and Coatings. Darocure-1173 photoinitiator was obtained from ESA Chemistry. Al2O3 nanoparticles were obtained from Evonik Industries AG.
Preparation of hybrid coatings
In this study, TMPTA containing two base formulations were prepared with or without MPTMS. The ratio of TMPTA to MPTMS was fixed at a molar ratio of 1:2. Other formulations were prepared by adding different amounts of PFOTES and Al2O3 nanoparticles.
Al2O3 nanoparticles were added to increase the roughness of the coatings. Darocure-1173 was used as a liquid type photoinitiator and its amount was fixed at a 1 wt%. The composition of all formulations is given in Table 1. Each formulation was prepared in a 100-ml beaker with adequate stirring until it became clear and homogeneous. To remove air bubbles formed during mixing, the beaker content was heated to 60 °C. After removal of air bubbles, hybrid-free films were prepared by pouring the light sensitive viscous liquid formulations on to a surface-modified Teflon™ mold. Beside that, to prevent the inhibiting effect of oxygen, the mixture in the well was covered by transparent, 100-μm-thick polyester film before irradiation with a high pressure UV-lamp (OSRAM, 300W). A quartz glass plate was also placed over the polyester film to obtain a smooth surface and uniform thickness. After 180-s irradiation under UV-lamp, hybrid-free films were annealed at 100 °C for 1 day to achieve high degree cross-linking via condensation of the alkoxysilane groups. MPTMS-containing films obtained after UV curing were flexible. After thermal treatment, the films became remarkably hard. Scheme 1 shows the structures of all the monomers used and also depicts the preparation of the photocurable coatings.
Characterization
Gel contents of the UV-cured films were determined by Soxhlet extraction for 6 h using acetone. Insoluble gel fraction was dried in vacuum oven at 40 °C to constant weight and the gel content was calculated.
Mechanical properties of the UV-cured free films were determined by standard tensile stress–strain tests to measure modules, ultimate tensile strength, and elongation at break. Standard tensile stress–strain experiments were performed at room temperature on a Materials Testing Machine Z010/TN2S, using a crosshead speed of 5 mm/min.
The wettability characteristics of free films were performed on a Kruss (Easy Drop DSA-2) tensiometer. The contact angles (θ) were measured by means of sessile drop test method in which drops were created using a syringe. Measurements were made using 3–5 μl of distilled water and hexadecane. For each sample, at least five measurements were made, and the average was taken.
TGA of the UV-cured free films were performed using a Perkin-Elmer Thermogravimetric analyzer Pyris 1 TGA model. Samples were run from 30 to 750 °C with heating rate of 10 °C/min under air atmosphere.
The LOI values of the coating materials were measured by using a Fire Testing Technology (FTT) type instrument, on the test specimen bar of 120 × 60 × 3 mm3 according to ASTM D2863-08.
SEM imaging of the films were performed on Philips XL30 ESEM-FEG/EDAX. The specimens were prepared for SEM by freeze-fracturing in liquid nitrogen and applying a gold coating of ~300 Å.
Results and discussion
To remove soluble fractions in UV-cured hybrid films, each sample was extracted with acetone. Gel content of the polymeric films was found to change between 99 and 98 % (Table 1). These high gel content values indicate high conversion of the UV-curable/sol–gel system and thus it can be concluded that strongly cross linked networks were formed. Also, the high gel contents indicate that MPTMS was successfully incorporated into the polymer matrix via thiol-ene click reaction. We must note that MPTMS was used without hydrolysis. We could not be able to prepare formulations containing higher amounts of fluorine due to the immiscibility of the monomers used. Thus, the aluminum oxide nanoparticles were added to F5 and F6 formulations. Also, the added amount of the aluminum oxide nanoparticles was found to be limited due to nanoparticle agglomeration. It was found that the addition of Al2O3 particles did not cause a decrease in the gel content values of the coatings. Therefore, it can be said that the nanoparticles are well entrapped in the polymeric network.
The hydrophobicity and the oleophobicity of the hybrid coating materials were investigated by water and hexadecane contact angle measurements. The results are shown in Table 2. The results showed that the first base formulation (F1) was slightly hydrophobic. Also, it can be seen that the addition of MPTMS did not result in a significant enhancement. On the other hand, it can be seen that the addition of fluorine significantly increases both the water and the hexadecane contact angles. This behavior of fluorine-containing materials is related with the segregation of the fluorinated segment on the surface of the coatings and as a result the surface energy of the coatings decreases [26]. Thus, the fluorine-enriched surface of the coatings showed increased hydrophobicity. When the fluorine content was doubled, water and hexadecane contact angles were only slightly increased. Likewise, the addition of Al2O3 nanoparticles was found to be ineffective on the hydrophobicity of the coatings. This situation was attributed to the relatively low content of Al2O3 particles. While these particles at those amounts were inefficient for hydrophobicity, they also worked against the oleophobic properties of the coatings. Overall, when compared with the base formulations, it can be said that the hydrophobic and oleophobic properties of the fluorine-containing coatings were greatly enhanced. In Fig. 1, there are eight different images that were taken from the Kruss software. Figure 1a, b, c, and d shows the water and hexadecane drops on the surface of the samples F2, F4, F6, and F7, respectively.
Tensile strength, elongation at break, and Young’s modulus of the hybrids are displayed in Table 3. From these results, it can be clearly seen that tensile modulus (300 MPa) of the second base formulation (628 MPa) is more than two times the modulus of the first formulation which is a result of the condensation of silane precursor. The addition of PFOTES also increased the tensile modulus of the coatings by introducing more cross-linking. Moreover, PFOTES increased the elongation at break values due to the flexible perfluoroalkyl groups. On the other hand, the addition of Al2O3 nanoparticles caused a decrease in the modulus of the coatings which can be related with the decreased condensation between alkoxysilane precursors. Also, PFOTES has a long aliphatic chain and these segments make the coating more flexible [27].
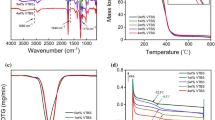
TGA technique was used to investigate the thermal oxidative stability of these organic–inorganic hybrid materials. TGA thermograms of the hybrids are given in Fig. 2. In Table 4, it can be seen that the first weight loss temperature was decreased when MPTMS was added to the first formulation. This situation was attributed to the release of remaining water, ethoxy and methoxy groups from the polymeric network. Furthermore, the addition of PFOTES and aluminum oxide nanoparticles were found to increase the first weight loss temperatures. The maximum weight loss temperatures were around 445 °C for all samples. The char yields at 750 °C were also collected. The introduction of fluorine and aluminum oxide nanoparticles improved the thermal properties of the coatings and resulted in char yields as high as 17.3.
LOI test is a commonly used method for the evaluation of the flame retardancy of materials. The LOI values of the hybrid-free films were found to increase as the amount of nanoaluminum oxide and fluorine was increased. Figure 3 shows the graph of the LOI values of the coatings. It can be seen from the plot that as the inorganic content of the coatings increases, the LOI values slightly increase. The LOI value for the base formulation (F1) was found as 19. But due to higher thermal stability of the C–F bonds in the fluoro acrylate, and the increasing amount of inorganic moiety, the LOI values increased. Yet the increase is not very large, due to the relatively small percent of the inorganic domain in the network.
Figure 4a–d shows the fractured surface morphology of the coatings. We can see from SEM micrographs that the base formulation F1 has a smooth and uniform surface without cracks. The introduction of MPTMS distorted the uniform structure of the surface and a layered surface structure was formed (Fig. 4b). Furthermore, the addition of PFOTES caused bulges on the fractured surface of the coatings. In both Fig. 4b and c, we can not see nanosilica formation. This situation is related with the in situ approach. In this study, neither MPTMS nor the PFOTES were hydrolyzed. Thus, the slow rate of hydrolysis and condensation reactions between the sol–gel precursors in the photo crosslinked polymer matrix prevented the formation of nanosilica particles. However, this situation did not cause a heterogeneous structure, and did not result in a reduction in the properties of the coatings. In Fig. 4d, it can be seen that the aluminum oxide inorganic particles were uniformly dispersed throughout the polymer matrix. Thus, it can be said that a homogenous hybrid network was formed successfully. Finally, it can be seen from the SEM images that the smooth surface of F1 becomes roughened as the fluorine and nanoparticles were incorporated into the polymer matrix.
Conclusion
The aim of this study was to prepare photocurable coatings using thiol-ene click reaction. An in situ approach was utilized for the preparation of hybrid coatings; thus, a fast preparation method was developed. MPTMS was successfully incorporated into the polymer matrix via thiol-ene click reaction and fluorine-containing photocurable coatings were prepared with high gel contents. The addition of fluorine and silica improved the mechanical properties, flame retardancy, hydrophobicity, and the oleophobicity of the coatings. The surface morphology of the hybrid films was characterized by SEM analysis. SEM studies indicated that inorganic particles were dispersed homogenously throughout the organic matrix. Aluminum oxide nanoparticles did not provide a major contribution to the properties of the coatings, only a slight increase was observed in the flame retardancy of the coatings. This was attributed to the relatively low amount of the Al2O3 nanoparticles.
References
Sirish KR, Cramer NB, Cross T, Raj R, Bowman CN (2003) Polymer-derived ceramic materials from thiol-ene photopolymerizations. Chem Mater 15:4257–4261
Zhang JY, Windall G, Boyd IW (2002) UV curing of optical fibre coatings using excimer lamps. Appl Surf Sci 186:568–572
Davidson RS, Holman RJ (2003) Developments and trends in radiation curing. Rev Prog Color 33:46–58
Han YH, Taylor A, Knowles KM (2008) Characterisation of organic–inorganic hybrid coatings deposited on aluminium substrates. Surf Coat Technol 202:1859–1868
Yano S, Iwata K, Kuri K (1998) Physical properties and structure of organic–inorganic hybrid materials produced by sol–gel process. Mater Sci Eng 6:75–90
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem 113:2056–2075
Zeng K, Guo M, Zhang Y, Qing M, Liu A, Nie Z, Huang Y, Pan Y, Yao S (2011) Thiol-ene click chemistry for the fabrication of Ru(bpy) 32+-based solid-state electrochemiluminescence sensor. Electrochem Commun. doi:10.1016/j.elecom.2011.08.004
Hoyle CE, Bowman CN (2010) Thiol-ene click chemistry. Angew Chem Int Ed 49:1540–1573
Posner T (1905) Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber Dtsch Chem Ges 38:646–657
Kharasch MS, Read J, Mayo FR (1938) The peroxide effect in the addition of reagents to unsaturated compounds. XVI. The addition of thio-glycolic acid to styrene and isobutylene. Chem Ind 55:752–773
Kumari S, Malvi B, Ganai AK, Pillai VK, Gupta SS (2011) Functionalization of sba-15 mesoporous materials using “thiol-ene click” Michael addition reaction. J Phys Chem C 115:17774–17781
Lowe AB (2010) Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem 1:17–36
Wendeln C, Rinnen S, Schulz C, Arlinghaus HF, Ravoo BJ (2010) Photochemical microcontact printing by thiol-ene and thiol-ene click chemistry. Langmuir 26:15966–15971
Yue J, Li X, Mo G, Wang R, Huang Y, Jing X (2010) Modular functionalization of amphiphilic block copolymersvia radical-mediated thiol-ene reaction. Macromolecules 43:9645–9654
Stamenovic MM, Espeel P, Camp WV, Du Prez FE (2011) Norbornenyl-based raft agents for the preparation of functional polymers via thiol-ene chemistry. Macromolecules 44:5619–5630
Turunc O, Meier MAR (2010) Fatty acid derived monomers and related polymers via thiol-ene (click) additions. Macromol Rapid Commun 31:1822–1826
Griesbaum K (1970) Problems and possibilities of the free-radical addition of thiols to unsaturated compounds. Angew Chem Int Ed 9:273–287
Hong LY, Cho YS, Kim DP (2005) Effect of component ratios on the performance of UV curing inorganic/organic coating. J Ind Eng Chem 11:275–279
Kim SW (2011) Characterization of UV curable hybrid hard coating materials prepared by sol–gel method. Korean J Chem Eng 28:298–303
Li F, Zhou S, Wu L (2005) Effects of preparation method on microstructure and properties of UV-curable nanocomposite coatings containing silica. J Appl Polym Sci 98:1119–1124
Chen CH, Ou MK, Lin SH, Tsai MS, Mao CF, Yen FS (2006) Preparation and application of an ultraviolet curable coating containing nanoscale α-aluminum oxide. J Appl Polym Sci 102:5747–5752
Bauer F, Flyunt R, Czihal K, Langguth H, Mehnert R, Schubert R, Buchmeiser MR (2007) UV curing and matting of acrylate coatings reinforced by nano-silica and micro-corundum particles. Prog Org Coat 60:121–126
Bauer F, Gläsel HJ, Decker U, Ernst H, Freyer A, Hartmann E, Sauerland V, Mehnert R (2003) Trialkoxysilane grafting onto nanoparticles for the preparation of clear coat polyacrylate systems with excellent scratch performance. Prog Org Coat 47:147–153
Bauer F, Flyunt R, Czihal K, Buchmeiser MR, Langguth H, Mehnert R (2006) Nano/micro particle hybrid composites for scratch and abrasion resistant polyacrylate coatings. Macromol Mater Eng 291:493–498
Choi SJ, Suh KY, Lee HH (2008) A geometry controllable approach for the fabrication of biomimetic hierarchical structure and its superhydrophobicity with near-zero sliding angle. Nanotechnology 19:275–305
Sheen YC, Huang YC, Liao CS, Chou HY, Chang FC (2008) New approach to fabricate an extremely super-amphiphobic surface based on fluorinated silica nanoparticles. J Polym Sci Part B 46:1984–1990
Kahraman MV, Bayramoğlu G, Boztoprak Y, Güngör A, Apohan NK (2009) Synthesis of fluorinated/methacrylated epoxy based oligomers and investigation of its performance in the UV curable hybrid coatings. Prog Org Coat 66:52–58
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çakmakçı, E., Mülazim, Y. & Kahraman, M.V. UV-curable fluorine-containing hybrid coatings via thiol-ene “click” reaction and an in situ sol–gel method. Polym. Bull. 70, 1037–1048 (2013). https://doi.org/10.1007/s00289-012-0871-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0871-2