Abstract
Random copolymer of ε-caprolactone with 2,2-dimethyltrimethylene carbonate has been synthesized by using N-heterocyclic carbene organocatalysts for the first time. A series of N-heterocyclic carbenes namely, imidazol-2-ylidenes substituted at 1,3 position by benzyl, isopropyl, ethyl and methyl were demonstrated to bring about the metal-free ring-opening copolymerization of ε-caprolactone and 2,2-dimethyltrimethylene initiated by benzyl alcohol in THF. The influences of reaction conditions, such as monomer, catalyst, and initiator concentration, as well as reaction temperature and time on the copolymerization have been examined in detail. The results show that 1-isopropyl-3-benzyl imidazol-2-ylidene carbene was potent organic catalyst for the ring-opening copolymerization of cyclic esters to generate well-defined linear polyesters of controlled molecular weight and narrow polydispersity. 1H NMR spectral data of copolymer obtained showed that the polymerization mechanism is in agreement with the activated monomer mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aliphatic polyesters have been extensively utilized in the biomedical field owing to their chemical stability, their solubility in both organic and aqueous media, their nontoxicity, their low immunogenicity, and antigenicity [1]. In this regard, the copolymer properties offering all the aforementioned advantages can be tailored by changing the composition of the copolymer required for different applications. Such materials can be synthesized with a high level of control through ring-opening polymerization of cyclic esters, and most often accomplished using various effective catalysts, including cationic, anionic, and coordination. However, the toxicity and difficulties in removal of the catalysts from the resulting polymer have limited their utilization in many cases [2–15]. Considering the disadvantages of the above catalysts, N-heterocyclic carbenes (NHCs) are a new kind of catalyst, and their nontoxicity, versatility, and chemical reactivity have received a considerable attention in molecular synthesis and more recently in macromolecular chemistry as well [16–21]. As a reliable alternative to metal-mediated catalyst, in particular, imidazolium-based carbenes represent a special class of NHCs that have emerged as powerful organocatalysts for polymer synthesis [18, 22, 23].
Copolymers of cyclic esters with ε-caprolactone (CL), 2,2-dimethyltrimethylene (DTC) are widely used as medical and environment friendly materials because of the biocompatibility and the biodegradability. In relation to this, various catalysts (cationic, anionic, coordination, etc.) have been developed for the ring-opening polymerizations. In particular, the imidazolium-based carbenes catalysts are inexpensive, highly active and nontoxic, as such, they provide an attractive method to yield polymers of well-defined molecular weight with narrow polydispersities (PDIs), characteristic of a polymerization system. In this article, we report the ring-opening copolymerization of CL and DTC using imidazol-2-ylidenes substituted at 1,3 position by benzyl, isopropyl, ethyl, and methyl as catalysts separately and present the relation between the structures and catalytic activity of imidazol-2-ylidenes, with emphasis on the polymerization characteristics and mechanism.
Experimental
Materials
ε-Caprolactone (Alfa Aesar, 99 %) was dried and distilled over fresh calcium hydride (CaH2) powder under reduce pressure, and stored over activated 4 Å molecular sieves at room temperature prior to use. 2,2-dimethyltrimethylene carbonate was synthesized according to a published method [24] and dried over P2O5. tetrahydrofuran (THF) was dried by refluxing over benzophenone–Na complex and distilled prior to use. Benzyl alcohol (BnOH) was refluxed over CaH2 for 48 h, distilled (in vacuo) and then stored over molecular sieves. All other materials were of analytical grade and were used as received.
Catalyst preparation
All catalyst preparations were carried out with Schlenk tube and vacuum-line technique under purified nitrogen. Imidazol-2-ylidenes (Scheme 1) were synthesized following the procedure described in the literature [15, 25, 26]. As the precursor of carbene catalysts, hydrophobic imidazolium hexafluorophosphates were prepared from corresponding halogen salts which slightly modified in literature [27–29].
Measurements
1H NMR and 13C NMR spectra were recorded on a Bruker AV-600 spectrometer in CDCl3 or DMSO-d6 with tetramethylsilane (TMS) as the internal reference at room temperature. Differential scanning calorimetric (DSC) curves were performed with a DSC (204F1) instrument. Each sample was first heated from −60 to 130 °C (first heating run) at a heating rate of 10 °C/min, held for 1 min to erase the thermal history, then cooled immediately to −60 °C at a cooling rate of 100 °C/min, and finally heated to 130 °C (second heating run) at 10 °C/min again under nitrogen. The number average molecular weight (Mn) and PDI measurements were obtained with THF as eluebt (1 mL/min) at 40 °C by gel permeation chromatograph (PL-GPC220) with refractive index detector and a set of columns (PL gel 10 μm MIXED-B 300 mm × 7.5 mm, PL gel 10 μm, Guard 50 mm × 7.5 mm) and calibrated using polystyrene standards.
Polymerization procedure
All copolymerization reactions were carried out in glass ampoules. Each ampoule was heated, evacuated, and filled with dry nitrogen several cycles before use. Monomer, solvent, initiator, and catalyst were added into the ampoules successively under a dried nitrogen atmosphere and kept thermostated. The reaction was quenched with a drop of water. The copolymer was precipitated from methanol and washed with methanol twice, and then dried to constant weight under vacuum at 40 °C.
Results and discussion
Characteristics of the polymerization
Table 1 lists the influence of three imidazol-2-ylidenes substituted by different groups as catalysts on the copolymerization of DTC with CL. The data show that their catalytic activities significantly depend on the steric and electronic effects of the catalyst. Particularly, when electron-donating ability of substituent on imidazol-2-ylidenes becomes greater, the catalytic activity is also increasing. The effect of introducing electron-donating substituent at 1,3 position of the imidazole ring on the copolymerization was examined. For example, the more electron-rich carbene (1) was highly effective catalyst for copolymerization of DTC with CL in comparison to (2) and (3), and produced high molecular weight copolymers (M n = 28,500 g/mol) with narrow polydispersities (PDI = 1.44) at room temperature in 60 min.
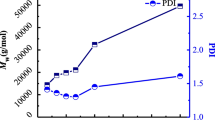
The influences of comonomer concentration, initiator amount, comonomer/catalyst molar ratio [DTC + CL]/[C], reaction temperature and time on copolymerization were examined with (1) as catalyst in THF. The results are summarized in Fig. 1 and 2, as well as Table 2. As seen in Fig. 1, the yield and the molecular weight of copolymer tend to rise with increasing comonomer concentration till 3.0 mol/L. But with higher concentration ([DTC + CL] > 3.0 mol/L), the comonomer conversion and the M n of copolymer decrease and the PDI broadens. Figure 2 illustrates that [DTC + CL]/[I] of 200 molar ratio is essential for preparing high yield and molecular weight copolymer. The copolymerization could not happen when further decreasing initiator content, yet increasing initiator content presumably leads to forming more and shorter copolymeric chains, thus decreasing the molecular weight of copolymer.
Comonomer/catalyst molar ratio [DTC + CL]/[C], reaction temperature, and time have a significant effect on the copolymerization, as shown in Table 2. From the results, it can be seen that the comonomer conversion, M n and PDI of poly (DTC-co-CL) can be controlled by varying factors mentioned above. Thus, the optimum conditions for the DTC and CL copolymerization ([DTC]/[CL] = 50:50) in THF are [DTC + CL] = 3.0 mol/L, [DTC + CL]/[I]/[C] = 200:1:0.4, 25 °C, 60 min.
The effect of the DTC/CL molar ratio in the feed on the composition, yield, and molecular weight of copolymer is shown in Table 3. It gives the results of the copolymerization with different DTC/CL molar ratio in feed. As can be seen, the higher content of CL in the feed, the higher conversion in copolymerization. While keeping comonomer composition unchanged, the copolymer produced always has a composition close to that in the feed.
Determination of reactivity ratios
Different compositions of copolymers were prepared at low conversion (10–15 %) for the purpose of determining the reactivity ratios of DTC and CL (Table 4). The composition of the copolymer can be calculated from 1H NMR spectrum (Fig. 6) by integrating the area of the peaks due to the methyl signals at δ1.0 (DTC unit) and the methylene signal at δ2.3 (CL unit). According to the Fineman-Ross Eq. (1), the reactivity ratios are calculated, respectively, as shown in Table 4.
where Y = (y − 1)x/y, X = x 2/y, y = d[DTC]/d[CL] in the copolymer, and x = [DTC]/[CL] in the feed.
The reactivity ratios, r DTC and r CL, are obtained from the slope and the intercept of the line in Fig. 3, and the values are 4.21 for r DTC and 0.25 for r CL. This indicates that DTC is favorable to incorporate into the propagating chains. The carbene (1) can attack the higher electrophilic carbonyl carbon of DTC easier than that of CL. The value of r DTC × r CL for the copolymerization catalyzed by carbene (1) is close to 1.0, indicating that the copolymerization is nearly ideal.
Characterization of the copolymer
GPC measurement showed a higher molecular weight and narrow molecular weight distribution (Fig. 4). Furthermore, the single peak of GPC pattern confirms that there is no homopolymer in the copolymerization reaction.
The random structure of copolymer can be demonstrated by 1H NMR and 13C NMR analysis. In the corresponding 13C NMR spectrum (Fig. 5), the typical chemical characteristic peak could be clearly identified at δ70.56 (DTC–DTC), δ64.33 (CL–CL), δ69.04 (DTC–CL), δ68.03 (CL–DTC), respectively, demonstrating the existence of copolymer (Scheme 2).
Figure 6 gives the 1H NMR spectra of the CH2–O region of copolymer as a function of reaction time. For the methylene groups, the signal at δ3.96 is found in DTC–DTC diad and CL–DTC diad. The signals are at δ3.90 for a DTC–CL diad, at δ4.06 for a CL–CL diad, and at δ4.12 for a CL–DTC diad. From the 1H NMR spectra, the compositions of the different diads were determined by integration, and the results are summarized in Table 5. The ratios of the diads DTC–DTC:DTC–CL:CL–DTC:CL–CL at various reaction times reveal that the concentrations of the heterodiads DTC–CL and CL–DTC increase with the copolymer yield, indicating that the copolymer structure becomes more and more random as the copolymerization proceeds
Analysis of the thermal properties of the copolymer by DSC is shown in Figs. 7 and 8. On the first heating, the copolymers exhibit some quite different melting transitions according to the molar ratio of DTC in copolymers. On the second heating, all the copolymers with various compositions show a single glass transition temperature (T g) between that of PCL (−65 °C) and PDTC (about −28 °C), confirming the random nature of the copolymers because block copolymer has two T g. When the content of CL unit in the copolymers is high, e.g., n(DTC)/n(CL) 20/80, it appears one melting transition which may result from the imperfect crystallites of the CL units.
Mechanism of 2,2-dimethyltrimethylene carbonate polymerization
The mechanism can be concluded that it involves an attack of the imidazol-2-ylidenes catalyst onto cyclic esters and the formation of a zwitterionic imidazolium alkoxide intermediate (activated monomer mechanism). According to the mechanism, initiation occurs when the nucleophile BnOH reacts with the monomer catalyst complex to form the ring-opened adduct. The α-chain end of the copolymer bears the ester from the initiating BnOH and the ω-chain end is a primary alcohol and serves as the nucleophile in subsequent propagation (Scheme 3).
Conclusion
We investigated an easy synthetic route to copolymer (DTC-co-CL), combining significant advantages, among which prevention of metallic initiators and residues, perfect control over molar masses and PDIs, absence of intermediate steps, and straightforward purification by precipitation. This could be achieved by using imidazol-2-ylidenes as an effective catalyst for the metal-free copolymerization of DTC and CL in THF at room temperature, in the presence of benzyl alcohol as initiator, preparing copolymer with high molecular weight and narrow molecular weight distribution. The mechanism can be proposed that the monomer is activated by the carbene, with propagation occurring by acyl-oxygen cleavage with alcohol present in solution. Propagation through chain extension of the alcohol species affords high molecular weight copolymers.
References
Thompson MS, Vadala TP, Vadala ML, Lin Y, Riffle JS (2008) Synthesis and applications of heterobifunctional poly(ethylene oxide) oligomers. Polymer 49:345–373
Rokicki G (2000) Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog Polym Sci 25:259–342
Yasuda H (2000) Organo transitionmetal initiated living polymerizations. Prog Polym Sci 25:573–626
Okada M (2002) Chemical syntheses of biodegradable polymers. Prog Polym Sci 27:87–133
Gerhard-Abozari E, Keul H, Hocker H (1994) Copolymers with soft and hard segments based on 2,2-dimethyltrimethylene carbonate and ε-caprolactone. Macromol Chem Phys 195:2371–2380
Hovestadt W, Keul H, Hocker H (1992) Tetraphenylporphyrin-aluminium compounds as initiators for the ring-opening polymerization of 2,2-dimethyltrimethylene carbonate: synthesis of homopolymers and copolymers with ε-caprolactone, ethylene oxide and propylene oxide. Polymer 33:1941–1948
Baiardo M, Alfonso GC (2001) Thermal and mechanical properties of diblock copolymers based on 2,2-dimethyltrimethylene carbonate and ε-caprolactone. Macromol Chem Phys 202:2509–2517
Akatsuka M, Aida T, Inoue S (1995) Alcohol/methylaluminum diphenolate systems as novel, versatile initiators for synthesis of narrow molecular weight distribution polyester and polycarbonate. Macromolecules 28:1320–1322
Kuhling S, Keul H, Hocker H (1992) Copolymerization of 2,2-dimethyltrimethylene carbonate with 2-allyloxymethyl-2-ethyltrimethylene carbonate and with ε-caprolactone using initiators on the basis of Li, Al, Zn and Sn. Macromol Chem Phys 193:1207–1217
Albertsson AC, Eklund MJ (1994) Synthesis of copolymers of 1,3-dioxan-2-one and oxepan-2-one using coordination catalysts. Polym Sci A 32:265–279
Kricheldorf HR, Stricker A (1999) Polylactones, 47. A-B-A triblock copolyesters and random copolyesters of trimethylene carbonate and various lactones via macrocyclic polymerization. Macromol Chem Phys 200:1726–1733
Joziasse CAP, Grablowitz H, Pennings AJ (2000) Star-shaped poly[(trimethylene carbonate)-co-(ε-capro-lactone)] and its block copolymers with lactide/glycolide: synthesis, characterization and properties. Macromol Chem Phys 201:107–112
Gupta AP, Kumar V (2007) New emerging trends in syntheticbiodegradable polymers – Polylactide: Acritique. Eur Polym J 43:4053–4074
Arduengo AJ, Dais RL, Harlow R, Kline M (1991) A stable crystalline carbine. J Am Chem Soc 113:361–363
Arduengo AJ, Dais RL, Harlow R, Kline M (1992) Mono(cclopentadienyl) titanium polymerization of ε–caprolactone. J Am Chem Soc 114:5530–5534
Arduengo AJ, Bertrand G (2009) Carbenes introduction. Chem Rev 109:3209–3210 (for an Editorial on Carbenes)
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Stable carbenes. Chem Rev 100:39–92 (for an Editorial on Carbenes)
Enders D, Niemeier O, Henseler A (2007) Organocatalysis by N-Heterocyclic carbenes. Chem Rev 107:5606–5655
Marion N, Díez-González S, Nolan SP (2007) N-Heterocyclic carbenes as organocatalysts. Angew Chem Int Ed 46:2988–3000
Hahn FE, Jahnke MC (2008) Heterocyclic carbenes: synthesis and coordination chemistry. Angew Chem Int Ed 47:3122–3172
Díez-González S, Marion N, Nolan SP (2009) N-Heterocyclic carbenes in late-transition metals catalysis. Chem Rev 109:3612–3676
Christmann M (2005) New developments in the asymmetric stetter reaction. Angew Chem Int Ed 44:2632–2634
Zeitler K (2005) Extending mechanistic routes in heterazolium catalysis-promising concepts for versatile synthetic methods. Angew Chem Int Ed 44:7506–7510
Sarel S, Pohoryles LA (1958) The stereochemistry and mechanism of reversible polymerization of 2,2-disubstituted 1,3-propanedil carbonates. J Am Chem Soc 80:4596–4599
Arduengo AJ, Krafczyk R, Schmutzler R, Craig HA, Goerlich JR, Marshall WJ, Unverzagt M (1999) Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 55:14523–14534
Kuhn N, Kratz T (1993) Synthesis of imidazol-2-ylidenes by reduction of imidazole-2(3H)-thiones. Synthesis 561–562
Bonhôte P, Dias A, Papageorgiou N, Kalyanasundaram K, Grätzel M (1996) Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem 35:1168–1178
Samantaray MK, Katiyar V, Pang KL, Nanavati H, Ghosh P (2007) Silver N-heterocyclic carbene complexes as initiators for bulk ring-opening polymerization (ROP) of l-lactides. Chem. 692:1672–1682
Thomas F, Mehran Y, John PR (2003) A structure-permeability study of small drug-like molecules. Bioorg Med Chem Lett 13:719–722
Acknowledgments
This study was supported by the Shanxi Natural Science Foundation of China (Grant No. 2006011069 and No. 2011011006-4), the Opening Foundation of Key Laboratory of Shanxi Province (Grant No. 2009011059-7), and the Research Fund for the Education Department of Shanxi Province (No. 2010111).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, R., Zhang, L., Wang, J. et al. Ring-opening copolymerization of ε-caprolactone with 2,2-dimethyltrimethylene carbonate using N-heterocyclic carbene organocatalysts. Polym. Bull. 70, 1289–1301 (2013). https://doi.org/10.1007/s00289-012-0854-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0854-3