Abstract
The maleated sulfur-prevulcanized natural rubber (M-SPNR) was prepared from grafting maleic anhydride (MA) onto SPNR latex particle by using benzoyl peroxide as an initiator. Natural rubber latex particle was vulcanized first, and then it was maleated to M-SPNR. The average particle size of M-SPNR was greater than that of SPNR possibly due to the formation of aggregate after addition of MA. The symmetric (strong) and asymmetric (weak) carbonyl stretching vibrations of succinic anhydride rings were confirmed by ATR–FTIR at 1,780–1,784 and 1,854 cm−1, respectively. The swelling ratios of M-SPNR latex film decreased with increasing MA contents. The tensile strength, modulus, hardness, and elongation at break of SPNR latex film dramatically increased after grafting with MA. Due to the reduction of double bond, the thermal stability of M-SPNR film was better than that of SPNR. The environmental friendly M-SPNR would be further applied as a compatibilizer between NR and biopolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural rubber (NR), composing mainly of cis-1,4 polyisoprene, is a biomaterial having good mechanical properties, i.e., green strength, tensile strength, and tear strength. However, their oil resistance, ozone resistance, and weather resistance are poor. These inferior properties of NR can be improved by the chemical modification, addition of additive, or blending with other polymers [1]. Besides the epoxidation [2–5], cyclization [6–8], hydrogenation [9, 10], chlorination [11–13], and grafting reactions [14, 15], the modification of NR with maleic anhydride (MA) has been studied [16]. With the aim of using, e.g., as compatibilizer, in the polymer reactive blending, NR in toluene was grafted with MA by using benzoyl peroxide (BPO) as a free radical initiator [16]. It was founded that the degree of grafted MA on NR chain (NR-g-MA) or maleated NR (MNR) was proportional to MA and initiator concentrations. An increase in the reaction time and reaction temperature caused an increase in the grafted MA content which, consequently, increased the glass transition temperature (T g) of MNR [17]. When adding the MNR in a polymer composite of NR and cellulose from paper sludge, the interfacial interaction between the two polymers was enhanced. The scorch time and cure time of the composite decreased with increasing filler loading. However, their maximum torque, viscous torque, and tan δ tended to increase [18]. Increasing paper sludge content also increased the modulus, whereas the tensile strength and elongation at break slightly decreased due to the reduction of rubber–filler interactions. In the case of NR/polyamide 6 blend, MA was added into NR prior to blending with polyamide 6 and the graft copolymer was formed during processing confirmed by rheology and thermal properties as well as dynamic mechanical analysis confirmed the grafting [19]. The morphology also showed a significant reduction in size of dispersed phase as MA was added into the rubber. It should be emphasized that the MNR was previously prepared from MA and NR in solution form. So, the main drawbacks of using organic solvent concerning environmental problem and high cost were still encountered.
In order to solve these problems, a green process in which the grafting reaction of MA onto NR in the latex form or water-based system was focused in this study. Moreover, we studied the grafting of sulfur-prevulcanized (SP) NR latex, generally used as a raw material in rubber manufacturing, with MA by using BPO which has not yet been reported. The application of maleated SPNR (M-SPNR) as a compatibilizer between NR and biopolymer was aimed to reduce the use of synthetic polymer in the future. The physical properties, i.e., tensile properties, dynamic properties, and thermogravimetric (TG) analysis, of M-SPNR were studied. Their particle size, zeta potential, and morphology were also investigated. The relationship between structure and properties of the M-SPNR was considered.
Experimental
Materials
High ammonia (HA)-NR (60 % dry rubber content (DRC) was supplied by Chalong Latex Industry Co., Ltd (Songkhla, Thailand). Poly(vinyl alcohol) with 89 % hydrolysis and vulcanizing agents, i.e., sulfur (S), zinc diethyldithiocarbamate (ZDEC), and zinc oxide (ZnO), were purchased from Sonal Company (Songkhla, Thailand). MA, BPO, and Butylated hydroxytoluene (BHT) or 2,6-bis(1,1-dimethylethyl)-4-methylphenol were supplied from Fluka (Seelze, Germany), Merck Schuchardt OHG (Seelze, Germany), and Kitpiboon Chemical Ltd (Bangkok, Thailand), respectively. Teric 16, a non-ionic surfactant and potassium persulphate (K2S2O8) were purchased from Lucky Four Company (Bangkok, Thailand) and Rankem analytical company (New Delhi, India), respectively. All chemicals were used as received.
Preparation of SPNR latex
The formulation used for preparation of SPNR latex is shown in Table 1. The vulcanizing agents, i.e., S, ZDEC, and ZnO in the form of 50, 10, and 50 % dispersion in water, respectively, were mixed with HA-NR latex. 10 % KOH and 25 % potassium laurate aqueous solution were then added into the mixture at 60 °C under stirring for 5 h.
Preparation of M-SPNR latex
MA (1.8 g) was dissolved in 10 % Teric 16 aqueous solution (10 mL), while BHT (0.0075 g) was dissolved in toluene (2 mL). Then, both MA solution (10 mL) and 1.5 % BHT solution (2 mL) were added in SPNR latex (50 mL; 30 % solid content) at 80 °C under stirring for 3 h. The resulting M-SPNR latex was poured into a glass plate (12 × 12 × 5 cm3) and allowed to dry at ambient temperature for 3 days.
Characterizations of SPNR and M-SPNR
Particle size and particle size distribution of the SPNR and M-SPNR latexes were measured by using a Laser Diffraction Particle Size Analyzer (Beckman Coulter laser LS™ 13 320). The zeta potential of SPNR latex particle was determined by using a microelectrophoresis apparatus (Zetasizer 4, Malvern) at 25 °C, pH 7. The M-SPNR latex film was characterized by attenuated total reflection mode Fourier-transform infrared spectrophotometer (ATR–FTIR) (Equimox 55, Bruker) for 100 times of scan. X-ray diffractometry (WI-RES-XRD-001, Philips X’Pert MPD) was performed under the following conditions: Nickel filtered Cu Kα radiation (λ = 0.15406 nm) at a current of 25 mA and a voltage of 35 kV. The scanning rate was 4°/min in the angle range of 5°–90° (2θ).
A known weight of M-SPNR film of specific dimensions (2.5 cm × 2.5 cm × 0.5 mm) was immersed in toluene for a period of 5 days at 32 °C. The sample was dried in an oven at 50 °C for 24 h and then weighed until constant weight. The swelling ratio was calculated from the Eq. (1) as follows:
where W 1 is the original weight of the sample, W 2 is the weight of the swollen sample
For TG analysis (TGA7, Perkin Elmer), each sample (6–7 mg) was analyzed under N2 with a flow rate of 45 mL/min. The temperature was varied from 50 to 800 °C at a heating rate of 10 °C/min. Five dumbbell test pieces of both M-SPNR and SPNR sheets from drying process were cut and the average thickness was calculated, and then attached between the grips of a tensile strength. The tensile strength and elongation at the break of both M-SPNR and SPNR sheets (Five dumbbell test pieces) were measured according to ASTM D412-98 at a crosshead speed of 500 mm/min with a load cell of 100 N. Modulus of the sample was also determined according to JIS K6251 using the tensile tester (Strograph E-L, TOYOSEIKI) at the same crosshead speed with the load cell of 500 N. The influence of aging at 90 °C on the M-SPNR and SPNR sheets was estimated from tensile strength.
Results and discussion
Particle size and zeta potential
SPNR latex was mixed with MA in the absence of Terric 16 and was then poured into a glass plate. The photograph obtained from a digital camera in Fig. 1A clearly showed the latex coagulation. It was explained that MA hydrolysed in water producing maleic acid which partially neutralized the negative charges, derived from indigenous non-rubbers (proteins and lipid), on SPNR particles [20, 21]. When MA was dissolved in aqueous solution of Terric 16 prior to mixing with SPNR latex, the stable colloid was observed as shown in Fig. 1B. It was reasonable that MA dispersed in aqueous solution of Terric 16 in the form of micelle as schematically presented in Fig. 2. The micelle having MA as core surrounding by Terric 16 molecules was loosely expanded in aqueous medium. When the MA occluded Terric 16 micelle collided with SPNR particle, MA could diffuse into SPNR particle [22, 23]. Therefore, Terric 16 molecules not only protected the direct contact between MA and aqueous medium or avoided the hydrolysis but also stabilized the M-SPNR latex particles by the steric effect [24]. This was similar to the stable epoxidized NR latex obtained at pH 4 from NR latex stabilized by a non-ionic surfactant (fatty alcohol/ethylene oxide condensate type) [24]. Results also showed that the latex stability depended on the DRC, surfactant concentration, and pH. In addition, the grafting of MA should occur on the surface of SPNR particle as shown in Fig. 2.
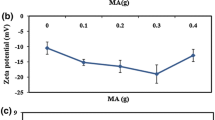
The data of particle size, particle size distribution, and zeta potential of the SPNR and M-SPNR are displayed in Fig. 3. It was observed that the average particle size of M-SPNR was greater than that of SPNR possibly due to the formation of aggregate after addition of MA as previously mentioned. When MA stabilized with Terric 16 was added into latex, the latex stability originally controlled by electrostatic effect was governed by the steric effect [25]. The presence of long chain non-ionic surfactant caused a slight increase in the hydrodynamic volume of latex and enlarged the shear plane of particle [26]. This explanation correlated well with the decrease of the absolute value of zeta potential of the M-SPNR latex.
FTIR and XRD
The possible chemical reaction between NR and MA is presented in Fig. 4. BPO decomposed into free radicals which then reacted with carbon–carbon double bonds of MA and NR molecules [16]. Besides the grafting of MA on cis-1,4-polyisoprene of NR, the crosslink product from NR molecules through MA bridging could be also obtained. Moreover, BPO radicals might break both SPNR and the M-SPNR molecules into short chains during the modification of SPNR. In addition, oxygen radical occurred from activation of oxygen with heat could react with double bond of SPNR and M-SPNR molecules [16].
Figure 5 shows the IR spectra of SPNR and of M-SPNR at the wavenumber of 4,000–500 cm−1. The absence of unreacted MA was indicated by the disappearance of the characteristic band at 698 cm−1 (C=C bond of MA) [16]. A broad and intense characteristic peak at 1,780–1,784 cm−1 and a weak absorption peak at 1,854 cm−1 due to the symmetric (strong) and asymmetric (weak) C=O stretching vibrations of succinic anhydride rings, respectively, were observed in the M-SPNR which supported the grafting of MA on SPNR [16]. The grafted MA was also deduced from the absorbance ratio of IR peaks at 1,780–1,784 and 1,854–835 cm−1 (–C–H stretching on cis C=C bonds of cis-1,4-polyisoprene). The absence of any peak at 1,850 and 1,780 cm−1 confirmed the absence of non-reacted MA.
The chemical reaction between SPNR and MA was also confirmed by XRD and the XRD patterns of the SPNR and M-SPNR are presented in Fig. 6. The broad XRD band indicated the amorphous state of SPNR. The hump at 2θ = 19° was attributed to the amorphous halo of SPNR at the same 2θ XRD pattern position [4]. On contrary, many crystallize patterns cited at 16°, 19.5°, 21°, 32°, 25°, 26°, 39°, and 40.5° were observed in the M-SPNR. After aging at 90 °C for 12 h, the partial crystalline patterns disappeared due to the loss of MA residue. From this evidence, it was concluded that SPNR was successfully grafted with MA by using BPO as an initiator.
Swelling ratio
The swelling ratio of the M-SPNR film immersed in toluene at ambient temperature for 5 days was used for confirmation of the chemical reaction between SPNR and MA. The swelling ratios of M-SPNR plotted with MA contents are shown in Fig. 7. It was found that the swelling ratio of the M-SPNR decreased from 3.7, 3.4, 3.3 to 3.2 when increasing MA content from 0, 3, 6 to 9 % w/w. It might be due to the increase in polarity of M-SPNR and also the possibility of crosslink of rubber chains through MA bridging as previously described.
Mechanical properties
The mechanical properties, i.e., hardness, tensile strength, modulus, and elongation at break, of the M-SPNR were investigated and the data are presented in Fig. 8. In Fig. 8A, it was clear that the hardness (shore A) of the M-SPNR was higher than that of SPNR and increased as a function of the MA contents. This result indicated the change of chemical structure of SPNR after addition of MA. Besides grafting of MA on SPNR, the hard sample might be caused from the crosslinked rubber chains through MA bridging. This explanation could be also used for the tensile strength of the M-SPNR in Fig. 8B which increased from 18, 20, 22, and 23 MPa with increasing MA contents from 0, 3, 6, and 9 % w/w. The modulus of the M-SPNR in Fig. 8C also correlated to their tensile strength. In addition, Fig. 8D shows the good elongation at break of the M-SPNR as compared with SPNR. However, the elongation at break of SPNR was constant at 576 %, while the elongation at break of M-SPNR in the presence of MA at 3, 6, and 9 wt% was 578.5, 579, and 579.5 %, respectively. This might be because the presence of MA and crosslinked chains in the M-SPNR played the major role.
Thermal properties
TGA thermograms of the SPNR and the M-SPNR are displayed in Fig. 9.
The data indicated that ~95 % of SPNR was decomposed at temperature between ~350 and 500 °C. The short polymer chains were, therefore, formed as a consequence of polymeric chain random scissions [27]. The weight loss from 450 to 500 °C of <10 % could be assigned to the thermal decomposition of the SPNR into dipentene and different unsaturated volatile products [27]. It was previously reported that a small peak observed in the DTG curve of NR at ~420 °C assigned to the crosslinked and cyclized rubbers [17]. The maximum weight loss of the SPNR curves was observed at 383 °C. In the case of M-SPNR, the thermal degradation started at 130 °C but completed at 470 °C. From 150 to 350 °C, the loss of 30 % of the M-SPNR might be due to the decomposition or chain scission of SPNR during the chemical reaction. Similar to the SPNR, the large portion of the M-SPNR was lost from ~350 to 500 °C and it was believed that the crosslinked chains during the preparation of M-SPNR were destroyed [17]. As previously reported, the chemical crosslinks between the MNR molecules occurred during Mooney test and the resistance to flow of the molten MNR was found. The increase in chemical interaction and intermolecular bonding or crosslinking by increasing MA concentration caused the increase in the Mooney viscosity and the high weight loss of the MNR confirmed with TGA analysis. Therefore, both chain scission and crosslinking could be occurred during the grafting of SPNR latex with MA.
Aging property
The influence of aging on the tensile strength and elongation at break of the SPNR and M-SPNR is, respectively, presented in Fig. 10A, B. It was observed that the tensile strength of both rubbers decreased after exposing at 90 °C. However, the thermal stability of the M-SPNR was higher than that of the SPNR and, hence, the slight change in tensile strength of the former was observed. After aging at 90 °C for 6 h, the tensile strength of M-SPNR decreased from the original value of 11 %, while 22 % was detected in the case of SPNR. The results agreed well with the TGA thermogram and it was implied that SPNR molecule reacted with MA. Figure 10A represents the influence of aging time on the tensile strength of the M-SPNR. It was observed that their tensile strength remarkably decreased as a function of aging time. The percent decrease of tensile strength of M-SPNR from 28, 35, 48, 67, 70, and 74 % was observed after aging at 90 °C for 6, 12, 24, 48, 72, and 96 h, respectively. This indicated the degradation of the samples caused mainly by chain scission leading to decrease of crosslink density on prolonged heating. The elongation at break of the SPNR and M-SPNR containing 6 % w/w MA increased after aging for 16 h since some chain scissions of SPNR molecules which occurred during preparing M-SPNR acted as plasticizer [28]. After that, elongation at break dramatically decreased from 525 to 250 or estimated to be about half of the initial value within 90 h of aging time as shown in Fig.10B. These results also correlated well with their tensile strength which was explained that the degradation of the samples led to the decrease of crosslink density.
Conclusion
The stable M-SPNR in water-based system was successfully prepared from grafting SPNR latex with MA in the presence of Terric 16 The particle size of the M-SPNR latex was greater than that of SPNR due to partial coagulation. The chemical reaction of MA and SPNR was confirmed by the appearance of a new band in ATR–FTIR spectrum at 821 cm−1, by many crystallize XRD patterns cited at 19.5° and 40.5° and by the decrease of swelling ratio when increasing MA contents. Due to reduction of unsaturation in SPNR, the M-SPNR showed good tensile strength, hardness, and thermal stability and, hence, would be further applied as a compatibilizer between NR and biopolymer.
References
Pukkate N, Kitai T, Yamamoto Y, Kawazura T, Sakdapipanich J, Kawahara S (2007) Nano-matrix structure formed by graft-copolymerization of styrene onto rubber. Eur Polym J 43:3208–3214
Riyajan S, Tanbumrung K, Pinyocheep P (2010) Physical properties of a ‘green’ polymer blend based on PVA starch and ENR. KGK-Kaust Gummi Kunst 63:371–376
Gan LH, Ng SC (1986) Kinetic studies of the performic acid epoxidation of natural rubber latex stabilized by cationic surfactant. Eur Polym J 22(1986):573–576
Riyajan S, Chaiponban S, Tanbumrung K (2009) Investigation of the preparation and physical properties of a novel semi-interpenetrating polymer network based on epoxised NR and PVA using maleic acid as the crosslinking agent. Chem Eng J 153:199–205
Johnson T, Thomas S (2000) Effect of epoxidation on the transport behaviour and mechanical properties of natural rubber. Polymer 41:7511–7522
Riyajan S (2009) Activation energy and thermodynamic parameters of cyclization in purified natural rubber latex using a trimethyl silyl triflate. J Elastom Plast 41:133–144
Riyajan S, Tuampoemsab S, Sakdapipanich JP (2008) Dynamic mechanical and physical properties of cyclized natural rubber blends. KGK-Kaust Gummi Kunst 61:665–670
Riyajan S, Sakdapipanich JT (2009) Thermal property and thermodynamic parameter of rubber blends containing the cyclic structure. KGK-Kaust Gummi Kunst 62:665–670
Mahittikul A, Prasassarakich P, Rempel GL (2009) Hydrogenation of natural rubber latex in the presence of [Ir(cod)(PCy3)(py)]PF6. J Mol Catal A 297:135–141
Simma K, Rempel GL, Prasassarakich P (2009) Improving thermal and ozone stability of skim natural rubber by diimide reduction. Polym Degrad Stabil 94:1914–1923
Ho CC, Khew MC (1999) Surface characterisation of chlorinated unvulcanised natural rubber latex films. Inter J Adhes Adhes 19:387–398
Krentsel LB, Travin SO, Litmanovich AD, Yutujan KK (1985) Initial stage of the chlorination of natural rubber. Eur Polym J 21:405–408
Kofman VL, Podmasterev VV, Razumovskii SD, Krentsel LB, Litmanovich AD (1987) Structure of products of the initial stage of chlorination of natural rubber. Polym Sci USSR 29:1224–1230
Kongparakul S, Prasassarakich P, Rempel GL (2009) Catalytic hydrogenation of styrene-g-natural rubber (ST-g-NR) in the presence of OsHCl(CO)(O2)(PCy3)2. Eur Polym J 45:2358–2373
Oliveira PC, Guimarães A, Cavaillé JY, Chazeau L, Gilbert RG, Santos AM (2005) Poly(dimethylaminoethyl methacrylate) grafted natural rubber from seeded emulsion polymerization. Polymer 46:1105–1111
Nakason C, Kaesaman A, Supasanthitikul P (2004) The grafting of maleic anhydride onto natural rubber. Polym Test 23:35–41
Nakason C, Saiwari S, Kaesaman A (2006) Rheological properties of maleated natural rubber/polypropylene blends with phenolic modified polypropylene and polypropylene-g-maleic anhydride compatibilizers. Polym Test 25:413–423
Ismail H, Rusli A, Rashid AA (2005) Maleated natural rubber as a coupling agent for paper sludge filled natural rubber composites. Polym Test 24:856–862
Carone E Jr, Kopcak U, Gonçalves MC, Nunes SP (2000) In situ compatibilization of polyamide 6/natural rubber blends with maleic anhydride. Polymer 41:5929–5935
Veyret R, Elaissari A, Marianneau P, Sall AA, Delair T (2005) Magnetic colloids for the generic capture of viruses. Anal Biochem 346:59–68
Rippel MM, Lee LT, Leite CAP, Galembeck F (2003) Skim and cream natural rubber particles: colloidal properties, coalescence and film formation. J Colloid Interface Sci 268:330–340
Afkhami A, Bahram M, Gholami S, Zand Z (2005) Micell-mediated extraction for the spectrophotometric determination of nitrite in water and biological samples based on its reaction with p-nitroaniline in the presence of diphenylamine. Anal Biochem 336:295–299
Abe M, Uchiyama H, Yamaguchi T, Suzuki T, Oginot K (1992) Micelle formation by pure nonionic surfactants and their mixtures. Langmuir 8:2147–2151
Bacn NV, Mihailov M, Terlemezyan L (1991) On the stability of natural rubber latex acidified by acetic acid and subsequent epoxidation by peracetic acid. Eur Polym J 27:557–563
Romero-Cano MS, Martin-Rodriguez A, Chauveteau G, De Las Nieves FJ (1998) Electrokinetic characterization of polystyrene–non-ionic surfactant complexes. Colloid Surf A 140:347–356
Thwala JM, Goodwin JW, Mills PD (2009) Electrokinetic studies of colloidal silica particles dispersed in non-aqueous media in the presence of a nonionic surfactant, dodecylhexaethylene glycol monoether (C12E6). Colloid Surf A 335:33–42
Martins MA, Moreno RMB, McMahan CM, Brichta JL, Gonçalves PDS, Mattoso LHC (2008) Thermooxidative study of raw natural rubber from Brazilian IAC 300 series clones. Thermochim Acta 474:62–66
Menon ARR, Pillai CKS, Nando GB (1998) Modification of natural rubber with phosphatic plasticizers: a comparison of phosphorylated cashew nut shell liquid prepolymer with 2-ethyl hexyl diphenyl phosphate. Eur Polym J 34:923–929
Acknowledgments
Research grant (RTA 5480007) from The Thailand Research Fund/the Commission on Higher Education is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riyajan, SA., Intharit, I. & Tangboriboonrat, P. Green preparation and physical properties of maleated sulfur-prevulcanized natural rubber. Polym. Bull. 69, 635–647 (2012). https://doi.org/10.1007/s00289-012-0746-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0746-6