Abstract
Microcapsules containing tetrachloroethylene as an internal phase were prepared by in situ polymerization of urea–formaldehyde (UF) without prepolymerization. The effects of different emulsifiers on the process of microencapsulation and morphology of microcapsules were investigated. The results show that the emulsifier gum arabic (GA) can effectively slow down the deposition rate of resin onto the oil/water interface, which can lead to smooth and compact surface of microcapsules. The surface activity of GA was also enhanced by complex formation of gum arabic and sodium dodecyl benzene sulfonate. The microcapsules represent good thermal and barrier property as a result of the formation of capsule wall with compact microstructure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microcapsules are used in various applications, such as pharmaceuticals [1, 2], dyes [3, 4], coatings [7], food additives [5, 6], catalysts [7, 8], energy storage [9, 10] and so on. In recent years, they are spotlighted as electrophoretic ink, which is used for a display element of Digital Paper [11]. To apply a microcapsule for an electrophoretic ink, it is preferable to use monodispersed small microcapsules having a thin membrane. One class of wall materials which is frequently used for microencapsulation is amino resins (especially resins of melamine and formaldehyde) which are low-cost and easy to handle. For microcapsules based on amino resins an in situ encapsulation process, which is described in literature [12–16], is used.
The traditional process for preparing poly(urea–formaldehyde) (PUF) microcapsule is based on the following two steps: First, preparation of precondensate: a mixture of urea and formaldehyde was adjusted to pH 8–9 with triethanolamine or NaOH and stirred at 70 °C for 1–1.5 h, the viscosity of the liquid increases and a linear formalin–urea polymer is formed. The product is water-soluble and in solution state. Second, encapsulation: the appropriate amount of precondensate is dissolved in some of deionized water and an oily core material is emulsified and dispersed in the solution under agitation. Acid is added to the dispersion to reduce the pH from 4 to 3.5. The dispersion is then agitated for about 3–4 h at a temperature of 45 to 50 °C. The precondensate becomes cross-linked, yielding a three-dimensional water-insoluble structure which precipitates and envelops the oil drops. The preparation process for PUF microcapsules has been reported in detail in many publications [17, 18].
Another process of preparing PUF microcapsules is letting urea and formaldehyde react under acidic conditions without preparing precondensate [19, 20]. Compared to the above-mentioned two-step process, we might call this process as a one-step process, which is time saving and easily operated as a result of absence of precondensating and no need to adjust pH carefully during the condensation period. In the one-step process, emulsifier plays an important role in the formation of the capsule membrane [21]. The common emulsifiers used in one-step process are negatively charged polyelectrolyte material, such as ethylene maleic anhydride copolymer, poly(areylic acid), methylvinyl ether maleic anhydride copolymer, and styrene maleic anhydride copolymer [22]. Although a capsule prepared by these emulsifiers has a smooth inner surface, its outer surface is rough. This certainly will influence the optical behaviors of the electronic ink microcapsules.
To improve the morphology property of microcapsule, we investigated the role of emulsifier on the formation of the microcapsules. The key parameters involving emulsifier type, the emulsifier concentration, and the complex formulation of emulsifier were investigated in detail.
Experimental part
Materials
Urea, formaldehyde (37% aqueous solution), ammonium chloride, sodium chloride, and resorcinol used as shell material were purchased from Shanghai Lingfeng Chemical Regents Factory, China. Tetrachloroethylene (TCE) used as core material was purchased from Shanghai Resin Plant. China. Sodium dodecyl benzene sulfonate (SDBS) (83% purity) and gum arabic (GA), used as emulsifier, were purchased from Shanghai Lingfeng Chemical Regents Factory, China. The polymer surfactant, poly(ethylene-alt-maleic anhydride) (poly(E-MA)), was purchased from Sigma–Aldrich Co. Ltd (China).
Microcapsule preparation and characterization
An aqueous solution containing urea as a monomer, resorcinol and ammonium chloride as a crosslinking agent and surfactant were used as the continuous phase. The dispersed phase was the TCE. Figure 1 shows the preparation process of the microcapsules. After the pH of the aqueous phase was adjusted to pH 3.5 with NaOH or HCl aqueous solution, O/W emulsions were formed using a homogeniser at the rotation speed of 500 rpm. Then, the polycondensation reaction was started by adding 37 wt% formalin solution. To ensure a slow and sufficient liquid/liquid phase separation, the emulsion was heated at a rate of 1 °C min−1 to the target temperature of 55 °C. After 4 h, the reaction was ended. The microcapsules that had formed were separated from aqueous solution with suction filtration (filter pore size is about 11 μm) and the microcapsules were collected.
Surface morphology of microcapsule was observed using a scanning electronic microscope (SEM, JSM-6360, FEI). Microcapsules were mounted on adhesive tape and ruptured with a razor blade for shell thickness measurement.
Optical microscopy (OM) was recorded with an optical microscope (XSP-8C, Changfang). Microcapsules size distribution was analyzed by image analysis software (Motic image plus 2.0). Mean diameter were determined from data sets of at least 250 measurements.
Thermogravimetric analysis (TGA) was performed with the TA SDT-Q600 from TA Instruments, under a nitrogen flow from 25 to 500 °C at a heating rate of 10 °C/min.
Results and discussion
Effect of the emulsifier type on the microcapsule formation
Emulsifier plays important roles in the preparation of microcapsules. With the help of emulsifier, the core materials emulsion can be stable and then the prepolymer is adsorbed and deposited on the surface of core particles. In this experiment, three emulsifiers, including Sodium dodecylbenzene sulfuric (SDBS), poly(ethylene-alt-maleic anhydride) (poly(E-MA)), GA, were used to improve the dispersion of the oil phase in the water phase. The concentration of emulsifiers in aqueous solution is 0.1, 0.4, 0.1 wt%, respectively. Under the same experimental conditions, they are all emulsified by stirring (500 rpm) for 30 min. The average droplet diameters for these systems containing 20 ml of TCE are presented in Fig. 2. It is found that using EMA and GA as emulsifiers yields large droplets with a broad size distribution, while using SDBS as emulsifiers yields small droplets with a narrow size distribution. These smaller droplet sizes are directly related to the fact that the O/W interfacial tension is decreased by a much greater extent when they are employed.
Figure 3 shows the optical micrographs of prepared microcapsules in SDBS, EMA, and GA. It is found that the influence of surfactant on the preparation of microcapsules is obvious. When no surfactant is used, microcapsules have irregular shapes as shown in Fig. 3a, which indicates that the core droplets in solution are not stable even with the aid of the mechanical agitation. The large amount aggregated PUF nanoparticles in solution indicate that it is difficult for PUF nanoparticles to deposit on the surface of microcapsule. Obviously, the surfaces of microcapsules are not compact, so the microcapsules are easily fractured during the washing process.
SDBS is a common emulsifier to prepare PUF microcapsules. Yuan et al. [23, 24] reported to successfully prepare PUF microcapsules with it by two-step process. However, there was no microcapsule generated in this case. The microcapsules prepared with SDBS were observed in the cocktail solution after appointed reaction time (Fig. 3b). The resultant cocktail solution was separated to oil and water phases after it was settled. This is because SDBS is destabilization in the reaction solution, which will cause the emulsion to break. The microencapsulation process of SDBS as an emulsifier was monitored by OM. Figure 4 shows a sequence of aliquot images along with the temperature and pH value. From the figure we found that the emulsion breaking occurs after the pH has dropped dramatically from initial conditions. It indicates that the dramatically drop in pH may be lead to the emulsion breaking. To confirm this, we prepared microcapsules at constant pH (pH = 3.5) conditions by drop-wise addition of NaOH and HCl. The emulsion breaking was not happening during this process, but the UF nanoparticles remained in suspension and did not deposit onto the microcapsule surface. The presence of suspended nanoparticles made the filtration process cumbersome and yields were low. This phenomenon accords well with the results reported by the Ref. [19]. Prepared microcapsules as mentioned above, however, were ruptured after drying process.
EMA has been used as surfactant in preparing PUF microcapsule in many literatures [19, 25, 26]. Different from traditional small molecular emulsifier such as SDBS, the macromolecular emulsifier EMA possesses stronger adsorbability and dispersibility in oil-in-water emulsion system. Figure 3c shows the OM micrograph of microcapsule prepared by using EMA as an emulsifier. The microcapsules have the clear “hedgehog” morphology (as shown in Fig. 3c, inset). It can be explained as follows: when the emulsion is formed, the hydrophilic end of a poly(ethylene-alt-maleic anhydride) molecule stretches out its carboxyl group toward the water phase but its oleophilic end is dissolved in the oil droplet interior. A chain of poly(ethylene-alt-maleic anhydride) molecules is very long and has a lot of carboxyl groups (–COOH) on it. With the polymerization, the carboxyl on a poly(ethylene-alt-maleic anhydride) molecule will catalyze polymerization and make UF polymer produce around its chain. Thereby, the “hedgehog” morphology around the microcapsule will be produced. The microcapsules rough outer surface will obviously influence the optical behaviors of the microcapsule when it used in electrophoretic display field. Meanwhile, the thick layer of porous UF caused agglomerations in solution and after filtration when the core material was a solution or resin and solvent.
GA is a weak polyelectrolyte that carries carboxylic groups, and microelectrophoretic measurements have shown that GA is negatively charged above pH 2.2, because the dissociation of the carboxylic groups is suppressed at low pH (<2.2) [27]. When GA was used as an emulsifier, the large size and hydrophilicity of polysaccharide moiety form viscous adsorbed layers at liquid–liquid interface [28]. On one hand, it can protect droplets against aggregation over a wide range of unfavorable conditions. On the other hand, this viscous adsorbed layer slow down the deposition rate of UF nanoparticles on the microcapsule surface, resulting in smooth and compact surface of the PUF wall shell as shown in Fig. 3d.
Based on above results, it is believed that GA is the optimal emulsifier for preparing microcapsules with smooth surface morphology.
Effect of the GA concentration on the properties of microcapsules
To investigate the influence of GA concentration on the formation of PUF microcapsules, microcapsules with different GA concentration were prepared. Figure 5 displays SEM pictures of capsules synthesized with GA concentration of 0.05, 0.1, and 0.2 wt%. It can be seen that the surface roughness of the capsules decreases with increasing the GA concentrations. When in low GA concentrations due to the viscosity of the oil–water interface is too low that UF nanoparticles’ precipitation rate on the surface of the capsules is so fast that lead to higher surface roughness of the wall. When in high GA concentration, the viscosity of the oil–water interface is so large that the nanoparticles can not deposit on the interface, microcapsules with a smooth and thin polymer wall are formed. However, after drying of the product the capsules are strongly agglomerated and collapsed. This means that a capsule wall is formed during the encapsulation, but in the drying process the wall is partially broken and the TCE is released. Thus, the capsule wall is not stable enough to endure stress in practical application. So there is an optimal GA concentration in preparing PUF microcapsule. In this study, GA concentration of 0.1 wt% is beneficial for preparing smooth surface and mechanically stable microcapsules.
Figure 6 shows the size distributions of microcapsules prepared with different GA concentrations (0.05, 0.1, and 0.2 wt%). Over the concentration investigated, the GA concentration has no significant influence on the microcapsule size distribution.
Complex formulation of GA and SDBS
Gum arabic is an effective emulsifier at low pH and high ionic strength. Nevertheless, the level of surface activity is actually rather low in comparison with typical low-molecule-weight emulsifiers. The performance problem of low-molecule-weight emulsifiers, however, relates to the major contribution of electrostatic interactions to the adsorbed layer structure. Combined with the low surface coverage, this makes the emulsions susceptible to destabilization under unfavorable environmental conditions. For instance, SDBS-based emulsions are sensitive to destabilization at low pH value, as mentioned above. In contrast, the large molecular size and predominant hydrophilicity of GA allows for the formation of a thicker stabilizing layer that is capable of protecting droplets against aggregation over a wide range of unfavorable conditions, such as low pH and high ionic strength. So its interest in exploiting the combined advantages of SDBS and GA as functional ingredients via the development of SDBS–GA complexes as emulsifier and stabilizer.
Figure 7 shows the size distribution of microcapsules prepared with surfactant containing mixture of 0.1 wt% GA and different concentrations of SDBS. When the SDBS concentration is below 0.133 wt% (SDBS: GA < 1.3), the microcapsule size distribution becomes narrow and the mean diameter of microcapsules decreases with the addition of SDBS (Fig. 8b). Further increase of SDBS concentration from 0.133 to 0.2 wt% (1.3 < SDBS: GA < 2) leads to the aggregation of the microcapsules (Fig. 8c). At 0.267 wt% (SDBS: GA > 2), most of the droplets cannot form stable microcapsules (Fig. 8d).
This phenomenon can be interpreted as follows: At low rations of SDBS to GA (approximately <1.3), the majority of the SDBS molecules may be complexed with the GA molecules. Due to bound SDBS, the conjugate is much more surface-active than GA on its own; hence the conjugate is able to achieve surface layer saturation at a much lower bulk concentration. At the same time, due to the bound GA, the adsorbed SDBS layer is protected against destabilization under unfavorable environmental conditions (e.g., heating, low pH, high electrolyte concentrations, etc.). At high ratios of SDBS to GA (>2), the system is probably destabilized by the self-association of SDBS, which is not complexes with the GA molecules. Most of the droplets cannot form stable microcapsules due to the ability of SDBS to desorb GA from oil droplets.
The experimental results show that composite emulsifier is more effect than simple emulsifier, and the optimal emulsifying effect could be obtained at the ratio of SDBS to GA less than 1.3. The presence of one or more GA molecules on the external surface of the complex at low SDBS: GA ratios may provide increased steric stabilization and may explain the remarkable pH stability of the complexes reported herein.
Stability of microcapsules
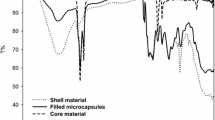
Figure 9 shows the TGA diagrams of PUF material and microcapsules prepared with 0.1 wt% of GA as surfactant. The gradual weight loss, not more than 30%, of microcapsules is found below the degradation temperature, due to the desorptions of small molecules such as H2O as well as the release of TCE. Since the range of the degradation temperature is much higher than the boiling point of tetrachoroethylene (121.2 °C). It is believed that microcapsules have good thermal property and the wall structure of microcapsules is compact.
The barrier property of the microcapsule, i.e., the ability of the capsule wall to protect core materials and prevent core materials from releasing outside, is important to the practical application. Figure 10 shows the weight loss of microcapsules at 50 °C. The weight loss was only less than 8% after heating for 10 h. It is obvious that microcapsules prepared with GA as emulsifier are stable and suitable for practical application.
Conclusion
Microcapsules containing TCE as an internal phase were prepared by in situ polymerization of UF without prepolymerization. The effects of different emulsifiers on the process of microencapsulation and morphology of microcapsules were investigated. The results show that the emulsifier gum arabic can effectively slow down the deposition rate of resin onto the oil/water interface, which can lead to smooth and compact surface of microcapsules. It is shown that the interfacial behavior of the phases in the system plays an important role in the process of microencapsulation, and a slow and sufficient liquid/liquid phase separation effected by an appropriate emulsifier under appropriate conditions is necessary for producing microcapsules of good morphology and membrane strength. The average diameter of microcapsules descends with the addition of complex formulation of GA and SDBS. Owing to the formation of capsule wall with compact microstructure, the microcapsules represent good thermal and barrier property, which are advantageous for the practical use of microcapsules in electrophoretic display.
References
Biju SS, Saisivam S, Rajan N, Mishra PR (2004) Dual coated erodible microcapsules for modified release of diclofenac sodium. Eur J Pharm Biopharm 58:61–67
Putney SD, Burke PA (1998) Improving protein therapeutics with sustained-release formulations. Nat Biotechnol 16:153–157
Sawada K, Urakawa H (2005) Preparation of photosensitive color-producing microcapsules utilizing in situ polymerization method. Dyes Pigments 65:45–49
Nelson G (2002) Application of microencapsulation in textiles. Int J Pharm 242:55–62
Shahidi F, Han X (1993) Encapsulation of food ingredients. Crit Rev Food Sci 33:501–547
Arshady R (1993) Microcapsules for food. J Microencapsul 10:413–435
Cho SH, White SR, Braun PV (2009) Self-healing polymer coatings. Adv Mater 21:645
Ji HB, Kuang JG, Qian Y (2005) Development of an immobilization method by encapsulating inorganic metal salts forming hollow microcapsules. Catal Today 105:605–611
Yu F, Chen ZH, Zeng XR (2009) Preparation, characterization, and thermal properties of microPCMs containing n-dodecanol by using different types of styrene-maleic anhydride as emulsifier. Colloid Polym Sci 287:549–560
Jiang YB, Wang DJ, Zhao T (2007) Preparation, characterization, and prominent thermal stability of phase-change microcapsules with phenolic resin shell and n-hexadecane core. J Appl Polym Sci 104:2799–2806
Comiskey B, Albert JD, Yoshizawa H, Jacobson J (1998) An electrophoretic ink for all-printed reflective electronic displays 394:253–255. doi:10.1038/28349
Dietrich K, Herma H, Nastke R, Bonatz E, Teige W (1989) Amino resin microcapsules. I. Literature and patent review. Acta Polym 40:243–251. doi:10.1002/actp.1989.010400406
Dietrich K, Bonatz E, Geistlinger H, Herma H, Nastke R, Purz HJ, Schlawne M, Teige W (1989) Amino resin microcapsules. II. Preparation and morphology. Acta Polym 40:325–331. doi:10.1002/actp.1989.010400507
Dietrich K, Bonatz E, Nastke R, Herma H, Walter M, Teige W (1990) Amino resin microcapsules. IV. Surface tension of the resins and mechanism of capsule formation. Acta Polym 41:91–95. doi:10.1002/actp.1990.010410205
Kage H, Kawahara H, Hamada N, Kotake T, Oe N, Ogura H (2002) Effects of core material, operating temperature and time on microencapsulation by in situ polymerization method. Adv Powder Technol 13:377–394. doi:10.1163/156855202320536025
Kage H, Kawahara H, Hamada N, Kotake T, Ogura H (2002) Operating conditions and microcapsules generated by in situ polymerization. Adv Powder Technol 13:265–285. doi:10.1163/156855202320252444
Park SJ, Shin YS, Lee JR (2001) Preparation and characterization of microcapsules containing lemon oil. J Colloid Interface Sci 241:502–508. doi:10.1006/jcis.2001.7727
Wang JP, Zhao XP, Guo HL, Zheng Q (2004) Preparation of microcapsules containing two-phase core materials. Langmuir 20:10845–10850. doi:10.1021/la0490902
Brown EN, Kessler MR, Sottos NR, White SR (2003) In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J Microencapsul 20:719–730. doi:10.1080/0265204031000154160
Fan CJ, Zhou XD (2009) Preparation and barrier properties of the microcapsules added nanoclays in the wall. Polym Adv Technol 20:934–939. doi:10.1002/pat.1342
Yoshizawa H, Kamio E, Hirabayashi N, Jacobson J, Kitamura Y (2004) Membrane formation mechanism of cross-linked polyurea microcapsules by phase separation method. J Microencapsul 21:241–249. doi:10.1080/02652040410001673946
Yoshizawa H, Kamio E, Kobayashi E, Jacobson J, Kitamura Y (2007) Investigation of alternative compounds to poly(E-MA) as a polymeric surfactant for preparation of microcapsules by phase separation method. J Microencapsul 24:349–357. doi:10.1080/02652040601162699
Yuan L, Liang GZ, Xie JQ, Li L, Guo J (2006) Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Polymer 47:5338–5349. doi:10.1016/j.polymer.2006.05.051
Yuan L, Gu AJ, Liang GZ (2008) Preparation and properties of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Mater Chem Phys 110:417–425. doi:10.1016/j.matchemphys.2008.02.035
Cosco S, Ambrogi V, Musto P, Carfagna C (2007) Properties of poly(urea-formaldheyde) microcapsules containing an epoxy resin. J Appl Polym Sci 105:1400–1411. doi:10.1002/app.26263
Blaiszik BJ, Caruso MM, McIlroy DA, Moore JS, White SR, Sottos NR (2009) Microcapsules filled with reactive solutions for self-healing materials. Polymer 50:990–997. doi:10.1016/j.polymer.2008.12.040
Ye A, Flanagan J, Singh H (2006) Formation of stable nanoparticles via electrostatic complexation between sodium caseinate and gum arabic. Biopolymers 82:121–133. doi:10.1002/bip.20465
Dickinson E (2009) Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocolloid 23:1473–1482. doi:10.1016/j.foodhyd.2008.08.005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, C., Zhou, X. Effect of emulsifier on poly(urea–formaldehyde) microencapsulation of tetrachloroethylene. Polym. Bull. 67, 15–27 (2011). https://doi.org/10.1007/s00289-010-0355-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-010-0355-1