Abstract
A soft template method for fabrication of polyaniline (PANI) microtubes is presented in this paper. It was found that methyl orange in acidic solution can be effectively self-assembled into supramolecular aggregates like flake and dendrite and they were used as soft templates to prepare PANI microtubes in the present of aniline monomer and ammonium peroxydisulfate. The morphology and chemical structure of the PANI microtubules were characterized by means of transmission electron microscopy, scanning electron microscopy, Fourier transformed infrared spectra, UV–vis absorption spectra (UV–vis) and elemental analysis. The results showed prepared PANI microtubes were in conductive emeraldine state.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent years, nano/microstructure conducting polymers have received great interest due to their promising applications in nanodevices [1], molecular electronics [2], microsensors [3], microactuators [4]. Among all the conducting polymers, polyaniline (PANI) has attracted extensive attention because of its many outstanding electrical and optical properties [5], as well as its ease of preparation, remarkable processability, excellent environmental stability and special electronic properties [6].
Nano/microstructure conducting polymer can be prepared by some methods such as template-synthesis [7], interfacial polymerization [8], electrospinning [9] and electrochemical polymerization [10]. Generally, the template synthesis, which involves hard template and soft template, is considered to be the most effective route to prepare nano/microstructure conducting polymers. The diameter of conducting polymer nanostructures was determined by the diameter of the pores in hard template [11]. Although inorganic aluminum oxide [12], zeolite with channels [13], and polymer membranes with porosity [14] were commonly used as the hard-templates, their preparation and the post processing for removing template was rather tedious, especially the oriented structures would be disturbed during the post-processes. The soft-template method is advantageous because an external template and post-treatment can be omitted [15].
Low-dimensional nanostructures of PANI in various shapes and forms such as nanoparticles [16], nanowires [17], nanotubes [18], nanofibers [19] and even spheres [20] have been successfully synthesized by soft-template method. However, there are few reports on the preparation of PANI microtubules by soft-template method, Micro-structure polymers have broad application prospect in mimetic enzyme, molecular reactor, smart capsule, load and release of drugs, biotic motor, through research on micro-structure of polymers can made great breakthrough in more fields. Here we report a facile approach to synthesize PANI microtubes in this article.
In past reports, researchers mostly used amphiphilic structure materials which contain alkyl hydrophobic group as dopant to prepare micro-structure polyaniline, amphiphilic structure material which contains azobenzene hydrophobic group was rarely reported. It was found that azo dye can form rodlike swarms at low azo dye concentrations in previous studies [21, 22]. Our previous experiment results showed polyaniline micro/nanotubes doped with novel dopant-acid mordant yellow GG were prepared by soft template method in the presence of ammonium persulfate (APS) as an oxidant. It was found that the [HCl]/[An] molar ratios and method of washing of the products played a key role in the formation of micro/nanotubes. Changing the molar ratio of HCl to aniline, the typical morphology of PANI could be changed from nanotubes to microtubes. By using the different method of washing of the products, there were significant changes in morphology of PANI micro/nanotubes.
In this experiment, it was found methyl orange (MO) in acidic solution could effectively self-assembled into supramolecular aggregates. By adjusting the molar ratio of MO to hydrochloric acid, supramolecular aggregates like flake and dendrite were obtained. Using them as soft templates in the polymerization, PANI microtubes were prepared with the inner diameter and outer diameter of about 800 nm and 1.5 μm respectively. The length of PANI tubes ranges from about 5–15 μm. The structural characters and the formation mechanism of PANI microtubes were also discussed.
Experimental
Materials
Aniline monomer (An) (Tianjin KRS Chem. Co.) was distilled under reduced pressure before using. APS, MO, hydrochloric acid were purchased from East China Chemical Reagents Factory and were used as received.
Characterization
The morphologies of products were investigated with a Philip XL-30E field emission scanning electron microscope (SEM) and a Philip Tecnai-20 transmission electron microscope (TEM). UV–vis spectra were measured in N,N-dimethyl formamide (DMF) on a Varian Cary-300 UV–vis spectrophotometer. Infrared spectra of PANI samples were conducted by means of a Bruker Vector-22 FT-IR spectrophotometers in the range of 4,000–400 cm−1. Elemental analyses of PANI samples were performed on a Thermo Flashea-1112 elemental analyzer. For conductivity measurements, the PANI samples were pressed into a round pellet 1 cm in diameter and analyzed using a four-probe instrument by applying a constant current.
Polymerization
A typical synthesis for the microstructure of PANI was as follows: aniline (5 mmol), MO (0.3 mmol) and a content hydrochloric acid was dissolved in 150 ml de-ionized water by supersonic stirring for 1 h at room temperature. Then, 50 ml aqueous solution of APS (5 mmol) was added to the above solution and the reaction was allowed to proceed in an ice bath with magnetic stirring for 12 h. Finally, the product was filtered and washed with distilled water, methanol, and ether each for several times and dried in vacuum at 40 °C for 24 h.
Results and discussion
In the experiment, the effect of the molar ratio of MO to hydrochloric acid was studied on morphology of the resulting PANI. It was found that the morphology of PANI was irregular grains (Fig. 1a) when the molar ratio was 0.1. But the morphology of PANI turned to regular rodlike (Fig. 1b) when the ratio was 0.06. In fact, typical TEM image (Fig. 2a, b) revealed that the rods were PANI microtubes. The inner and outer diameter of PANI tubes were about 800 nm and 1.5 μm respectively, the length of PANI tubes ranged from about 5–15 μm. Interestingly, in the absence of aniline monomers and APS, a little mixture from reaction system was taken and the morphology of the mixture was found different. When the molar ratio of MO to hydrochloric acid is 0.1, the morphology of mixture is flake (Fig. 3a). The morphology of mixture is dendrite (Fig. 3b) when the molar ratio is 0.06. It suggests that MO in acidic solution can effectively self-assembled into supramolecular aggregates. The morphology of PANI could be easily controlled by adjusting the molar ratio of MO to hydrochloric acid. It seemed that the ratio is a key factor for the formation of different supramolecular aggregates.
Although MO is not a typical surfactant, MO has a built-in amphiphilic design with hydrophilic sulfonic group and hydrophobic long alkyl chain. MO in different system may exist in more than one form of supramolecular aggregates like flake and dendrite. The polymerization will proceed with the addition of APS. In addition, APS as oxidant is water-soluble, polymerization would take place at the interface of template/water [23]. So these formed supramolecular aggregates like flake and dendrite may act as soft templates in polymerization. Finally, oxidative polymerization of aniline is confined by the supramolecular aggregates. Then PANI tubes were obtained by an elongation process [24].
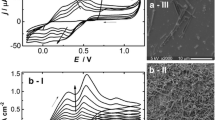
Figure 4 is the UV–vis absorption spectra of PANI irregular grains and PANI microtubes. In spectra, three peaks were observed around 330, 420 and 620 nm. The peak at 330 nm can be attributed to a π–π* benzenoid transition in the leucoemeraldine segments [25]. The broad peak at 620 nm corresponds to π–π* quinonoid transition in the pernigraniline segments [26].The peak at 420 nm is related to the polaron band–π* transition [27]. The polaron band transition around 420 nm is a typical absorption peak of doped PANI, this result reveals that prepared PANI is in doped state [28]. Compared to PANI irregular grains, absorption peak around 420 nm of PANI microtubes is broader and shifts to a higher wavelength which due to higher doping level of PANI microtubes.
The FTIR spectra of PANI irregular grains and PANI microtubes are shown in Fig. 5. For PANI microtubes, the peaks in the 3,450 cm−1 correspond to the N–H vibrations of the leucoemeraldine component [29]. The strong peaks at 1,584 and 1,495 cm−1 correspond to the C=C stretching modes of the quinonoid rings and benzenoid rings in the polyaniline chain, respectively [30]. The peaks at 1,251 and 1,304 cm−1 can be assigned to the C–N stretching mode of the polaron units [31]. And the peak at 509 cm−1 ascribed to the C–N–C torsion is also observed [32]. The benzene C–H bending deformation band at 1,142 cm−1 is characteristic of the semiquinone structure [33]. The band at 813 cm−1 is related to the C–H deformation, the band at 618 cm−1 is attributed to the benzenoid ring deformation [34]. The peaks at 1,042 and 699 cm−1 are relative to the S=O and S–O stretching vibration modes of the sulfonate groups attached to the aromatic rings [35]. For PANI irregular grains, similar result can be observed. But some differences between PANI microtubes and irregular grains are also observed. For PANI microtubes, the band at 1,142 cm, assigned as the doped state of PANI, is stronger than that of the PANI irregular grains. The results indicated that the doped level of PANI microtubes is higher and which were accord with the results of UV–vis spectra.
The results of elemental analysis for PANI samples are shown in Table 1. The S:N molar ratios of PANI microtubes and irregular grains are 0.11 and 0.09, respectively, which also indicated the doped level of PANI microtubes is higher. The result is consistent with its conductivity, UV–vis spectra and FTIR spectra. Due to the low doping level of the products, the conductivities of the as-prepared PANI are not high.
Conclusion
A facile method to synthesize PANI microtubules was reported. It was found that MO in acidic solution can form different supramolecular aggregates. By simply changing the molar ratio of MO to hydrochloric acid, supramolecular aggregates like flake and dendrite could be obtained. Two different morphologies of PANI with microtubes and grains were obtained by a polymerization process in the presence of supramolecular aggregates as soft templates via self-assembly process. The proposed method provides a novel route to design and synthesize PANI microstructures by soft template strategy, which may be applied to the synthesis of other nano/microstructures polymer.
References
MacDiarmid AG (1997) Synth Met 84:27
Ghosh P, Siddhanta SK, Chakrabarti A (1999) Eur Polym J 35:699
Morrin A, Ngamna O, Killard AJ, Moulton SE, Smyth MR, Wallace GG (2005) Electroanalysis 17:423
Somani P, Mandale AB, Radhakrishnan S (2000) Acta Mater 48:2859
Pang SP, Li GC, Zhang ZK (2005) Macromol Rapid Commun 26:1262
MacDiarmid AG (2001) Angew Chem Int Ed 40:2581
Martin CR (1995) Acc Chem Res 28:61
Sawell DD, Villahermosa RM, Lipeles RA, Hopkins AR (2004) Chem Mater 16:1606
Pinto NJ, Carrion P, Quinones JX (2004) Mater Sci Eng A 366:1
Liang L, Liu J, Windisch CF, Exarhos GJ, Lin YH (2002) Angew Chem Int Ed 41:3665
Zhang ZX, Deng J, Sui J, Yu L, Wan MX, Wei Y (2006) Macromol Chem Phys 207:763
Martin CR (1994) Science 266:1961
Wu GG, Bein T (1994) Science 264:1757
Wang Z, Chen MA, Li HL (2002) Mater Sci Eng A 328:33
Wei ZX, Zhang L, Yu M, Yang Y, Wan MX (2003) Adv Mater 15:1382
Li D, Kaner RB (2006) J Am Chem Soc 128:968
Liu H, Kameoka J, Czaplewski DA, Craighead HG (2004) Nano Lett 4:671
Wei ZX, Zhang Z, Wan MX (2002) Langmuir 18:917
Virji S, Huang J, Kaner RB, Weiller BH (2004) Nano Lett 4:491
Wei ZX, Wan MX (2002) Adv Mater 14:1314
Imae T, Gagel L, Tunich C, Platz G (1998) Langmuir 14:2197
Imae T, Ikeda S (1983) Mol Cryst Liq Cryst 101:155
Zhang L, Wan MX (2003) Adv Funct Mater 13:815
Harada M, Adachi M (2000) Adv Mater 12:839
Zhang L, Wan MX (2005) Thin Solid Films 477:24
Zhang L, Wan MX (2002) Nano tech 13:750
Fernandes MR, Garcia JR, Schultz MS, Nart FC (2005) Thin Solid Films 474:279
MacDiarmid AG, Epstein AJ (1995) Synth Met 69:85
Li G, Zhang Z (2004) Macromolecules 37:2683
MacDiarmid AG, Chiang JC, Halpern M, Huang WS, Mu SL, Somasiri NL, Wu W, Yaniger SI (1985) Mol Cryst Liq Cryst 121:173
Karatchevtseva I, Zhang Z, Hanna J, Luca V (2006) Chem Mater 18:4908
Cochet M, Louarn G, Quillard S, Buisson JP, Lefrant S (2000) J Raman Spectrosc 31:1041
Yan Y, Yu Z, Huang YW, Yuan WX, Wei ZX (2007) Adv Mater 19:3353
Liu H, Hu XB, Wang JY, Boughton RI (2002) Macromolecules 35:9414
Stejskal J, Sapurina I, Trchova M, Konyushenko EN, Holler P (2006) Polymer 47:8253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, L., Li, K. & Chen, X. Soft template method to synthesize polyaniline microtubes doped with methyl orange. Polym. Bull. 63, 15–21 (2009). https://doi.org/10.1007/s00289-009-0076-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-009-0076-5