Abstract
In the present study, the impact of co-inoculation of arbuscular mycorrhizal fungi (AM Rhizophagus sp., NCBI-MN710507) and Zinc solubilizing bacteria (ZSB2- Bacillus megaterium, NCBI-KY687496) on plant growth, soil dehydrogenase activity, soil respiration and the changes in bacterial diversity in rhizosphere of turmeric (Curcuma longa) were examined. Our results showed that higher plant height and dry biomass were observed in treatments co-inoculated with AM and ZSB2. Likewise, dehydrogenase activity and soil respiration were more significant in the co-inoculation treatment, indicating abundance of introduced as well as inherent microflora. Bacterial community analysis using 16S rRNA revealed changes in the structure and diversity of various taxa due to co-inoculation of AM and ZSB2. Alpha diversity indexes (Shannon and Chao1) and beta diversity indexes obtained through unweighted unifrac approach also showed variation among the treated samples. Chloroflexi was the dominant phylum followed by Proteobacteria, Actinobacteria and Acidobacteria which accounted for 80% of all treated samples. The composition of bacterial communities at genus level revealed that co-inoculation caused distinct bacterial profiles. The Linear discriminant analysis effect size revealed the dominance of ecologically significant genera such as Bradyrhizobium, Candidatus, Pedomicrbium, Thermoporothrix, Acinetobacter and Nitrospira in treatments co-inoculated with AM and ZSB2. On the whole, co-inoculated treatments revealed enhanced microbial activities and caused significant positive shifts in the bacterial diversity and abundance compared to treatments with sole application of ZSB2 or AM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Turmeric (Curcuma longa L.) is a crop of global importance and is well known for its anticarcinogenic, antimutagenic, antioxidant and chemotherapeutic properties. The plant contains curcumin which is valued in both traditional as well as modern medicines and has wide applications in pharmaceutical and cosmetic industries. Due to higher demand in world-wide markets, turmeric production and area is expanding over a decade. However, the non-availability of bioinoculants is one of the major constraints during turmeric cultivation [1]. In the present-day farming, bioinoculants are a very attractive proposition since it can substantially reduce the use of chemical fertilizers and pesticides. Hence, there are now an increasing number of inoculants being promoted for a number of crops. The understanding of the effects of inoculated bacterial and fungal strains on the inherent microbial communities continues to be of great interest since soil inoculation may lead to alterations in the composition of the inherent microbial communities [2] due to direct (antagonistic/ synergistic interactions) or indirect effects (enhanced root growth and exudation). Apparently, such changes in the microbial community structure following microbial inoculation might possibly result in trophic competition with indigenous microbial populations or mutually benefiting effects [3] which could produce additive or synergic impacts.
In global ecosystems, mutualistic associations between AM and host roots are nearly ubiquitous, with almost 90% of all terrestrial plants forming mycorrhizal colonization in their roots. AM colonization improves the uptake of plant mineral nutrients, primarily P and micronutrients, in exchange for photosynthetically fixed C which ultimately supports in growth and also develops tolerance to biotic and abiotic stresses in several plant species [4, 5]. The impact of AM on soil C cycling has also gained world-wide significance owing to the vital role played in terrestrial C fluxes [6]. Studies showed that the multifunctional services provided by AM are the result of the synergistic activity of various bacterial communities existing with their spores and extraradical mycelium and playing diverse plant growth-promoting roles, from fixation of N2 [7, 8], production of IAA, siderophores, antibiotics, soil P, K, Zn, Fe solubilization [9, 10]. Besides, AM and mineral solubilizing bacteria can act synergistically and enhance the amount of soil accessible nutrients resulting in increased uptake of nutrients, growth and yield of crop plants [11, 12]. Bacterial and fungal inocula together with organic nourishment could be an appropriate tool for refining soil quality and such a approach may help in design a eco- friendly integrated soil nutrient management to meet the world-wide food demand. Most of bioinoculants frequently depend on the application of a single strain which might partly account for the recorded variations in the field. An approach to overcome this issue is to take account of different species or strains of favourable microbes in the same bioinoculant formulation. Reports suggest that co-inoculation of bacteria and fungi could be a powerful approach for sustainable soil quality management [13, 14]. Consistent with these reports, combined inoculation of AM and Bacillus spp has been found to be a proficient method to increase plant growth [14]. Apparently, such combined application of microbial inoculants can manipulate, at least for the time being, the local bacterial communities. Therefore, the major concern regarding how the impact of co-inoculation of AM and beneficial bacteria on the structure and composition of bacterial communities and their functional abilities still remains unanswered. We hypothesized that combined application of AM and a promising Zn solubilizing bacteria (ZSB2) will impact dehydrogenase activity and soil respiration due to changes in the native bacterial community composition and structure in turmeric rhizosphere. To test this hypothesis, we performed high-throughput 16S rRNA gene amplicon sequencing to understand changes in composition and structure of the bacterial community in response to co-inoculation of AM and ZSB2 in turmeric rhizosphere.
Materials and Methods
Bioinoculant Preparation
Zinc solubilizing bacteria, Bacillus megaterium (ZSB2, NCBI-KY687496) was grown in LB broth up to 1010 cells mL−1 and then harvested by centrifugation at 6000 rpm for 10 min at room temperature. The cell pellets were washed twice with phosphate buffer (pH 7.4) and mixed uniformly in 2.0 mL of phosphate buffer and given as drenching near the root region. The mycorrhizal fungi, Rhizophagus sp. (AM, NCBI-MN710507) contained 100 infective propagules per gram of the inoculum in the form of spores, mycorrhizal roots and soil hyphae were prepared with vermiculite as the carrier.
Green House Experiment
Plastic bags having a capacity of 1.0 kg were selected for the pot culture experiment. The soil used in the study was a Typic humitropept with pH 4.08, EC 0.14 dSm−1, organic carbon 1.63%, available phosphorus 2.2 kg ha−1and available potassium 92 kg ha−1. Unsterilized soil was used in this experiment to evaluate the co-inoculation effects under natural conditions [15]. Healthy rhizomes (2.0 g) of turmeric (variety: Prathiba) were used with at least one sprouted buds were placed around 5 cm deep in each pot and covered with soil. 2.0 g of AM inoculum and 2.0 mL of ZSB2 were applied alone or in combination as per the treatments. The treatments were as follows: T1: Zinc phosphate + ZSB2; T2: Zinc phosphate + ZSB2 + AM; T3: Zinc phosphate + AM; T4: Zinc sulphate; T5: Absolute control. To assess the performance of bioinoculants on turmeric plant, parameters such as plant height, dry weight and AM colonization were recorded at 150 days after planting (DAP).
Soil Analysis
Rhizome initiation during the 5th month (150 DAP) is the most crucial and metabolically active stage in turmeric. During this stage, the plants were uprooted carefully and the soil adhering to the root was separated in a sterile petri dish and mixed thoroughly for stored for − 20 °C and room temperature for DNA extraction and other analysis.
Soil Respiration and Soil Dehydrogenase
For determining respiration, moist soil samples (50 g, 60% field capacity) were incubated in an airtight jar with a beaker containing 10 mL 0.5 M NaOH for 10 days. The released CO2 was calculated by titration of excess NaOH with 0.25 N HCl after adding of BaCl2. The concentration of CO2-carbon was expressed as mg CO2-carbon kg−110 days−1 [16]. Dehydrogenase activity was determined using 2,3,5-triphenyl tetrazolium chloride (TTC) as the substrate and measuring the intensity of triphenyl formazan in methanol extract using a spectrophotometer [17].
AM Colonization
AM colonization was estimated in AM inoculated soils by microscopical examination at 10× magnification, after clearing of roots with 10% KOH and staining with 0.05% trypan blue [18].
Bacterial Community Analysis
The DNA was isolated from soil samples using the DNeasy Power Soil kit (Qiagen, USA) from each soil as per the manufacturer's protocol. The DNA concentration was estimated using Qubit Fluorimeter (V.3.0). The V3-V4 region of 16S rRNA was amplified using specific primers (Forward primer: CCTACGGGNBGCASCAG and reverse primer: GACTACNVGGGTATCTAATCC) [19]. The amplified product was checked on 2% agarose gel and gel purification was done to remove non-specific amplifications. 5 ng of amplified product was used for library preparation using the NEBNext Ultra DNA library preparation kit. The library was prepared by using Agilent 2200 Tape Station and it was sequenced for paired-end sequencing (2 × 270 bp) on an Illumina HiSeq 2500 platform (Illumina, CA, USA). The microbiota profiling of samples was performed by sequencing the V3–V4 region of the 16S rDNA gene for bacteria using QIIME [20]. Quality checking, filtering and identification merging of V3–V4 region, OTU picking and assigning taxonomies have done at 97% identity using SILVA reference database [21, 22]. The sequence data obtained from the soil samples of turmeric rhizosphere were deposited in the in GenBank Sequence Read Archive with BioSample ID SAMN15905274, SAMN15905292, SAMN15905293, SAMN15905307 and SAMN15905309. (BioProject ID: PRJNA659126).
Statistical Analysis
Alpha diversity indexes (Shannon index and Chao1 index) were calculated to study the differences within the samples. Jackknife test was made to construct a consensus unweighted pair group method with arithmetic mean (UPGMA) tree for all samples to study the beta diversity indices. Linear discriminant analysis (LDA) together with effect size measurements (LEfSe), Kruskal–Wallis and pairwise Wilcoxon tests were carried out to measure the effect size of each distinctly abundant taxa [23]. All the treatments were replicated five times and one-way ANOVA was employed to test the significance among treatments. Post hoc comparison of means was then done using the least significant difference (LSD) test using the SAS 9.3 software.
Results
Bioinoculant Application on Growth and Biomass of Turmeric Plant
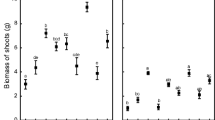
Plants inoculated with AM and ZSB2 showed enhanced growth (81.4 ± 9.6 cm) and dry weight (8.9 ± 0.8 g) of the turmeric through colonization of their adventitious roots as well as nutrient mobilization (Fig. 1a, b). AM could effectively infect the roots of turmeric and AM structures such as arbuscules, vesicles and hyphae were witnessed in all the treatment samples inoculated with AM fungi (Fig. S1).
Effect of bioinoculant application on (a) plant growth and (b) dry weight of turmeric plants under green house conditions. Values are means ± SE (n = 5); different letters indicate significant difference at LSD (0.05P). (T1: Zinc phosphate + ZSB2; T2: Zinc phosphate + ZSB2 + AM; T3: Zinc phosphate + AM; T4: Zinc sulphate; T5: Absolute control)
Soil Dehydrogenase Activity and Soil Respiration
Dehydrogenase (DH) activity was observed at 150 days after the bioinoculant application. Results showed that combined application ZSB2 and AM (0.98 ± 0.08 μg TPFg−1 h−1) significantly enhanced DH activity (Fig. 2a). In fact, in the co-inoculated treatment, DH activity was greater by 133% compared to control (absolute), by 117% compared to Zinc sulphate alone, by 34% compared to AM alone and 69% compared to only ZSB2.
Effect of bioinoculant application on (a) dehydrogenase activity and (b) soil respiration in turmeric rhizosphere under green house conditions. Values are means ± SE (n = 5); different letters indicate significant difference at LSD (0.05P). (T1: Zinc phosphate + ZSB2; T2: Zinc phosphate + ZSB2 + AM; T3: Zinc phosphate + AM; T4: Zinc sulphate; T5: Absolute control)
Inoculation with AM or ZSB2 alone did not increase respiration, whereas combined inoculation of AM with ZSB2 resulted in higher soil respiration (16.6 ± 1.6 mg CO2 100 g soil−1) (Fig. 2b). Compared to absolute control, the respiration level was greater by 110%, compared to 54% and 27.0% in treatments with only ZSB2 and AM, respectively.
Bacterial Community Structure
The rarefaction curves tended to attain the saturation plateau showing that the microbiota of the five treatment samples was large enough to estimate the microbial community diversity at the 97% similarity thresholds (Fig. S2). All samples were normalized for downstream analyses in QIIME and each experimental sample received more than 30,000 valid reads. A total of 42,131 OTUs were identified from 1,841,617 reads. A total of 42,131 OTUs were identified and after removing the less reads, 9396 OTUs were selected for downstream analysis.
The results on alpha diversity indicated that there was greater evenness, diversity and richness in the treatments with sole inoculation of ZSB2 and AM, while the treatment involving co-inoculation of AM with ZSB2 registered lower richness and diversity (Fig. S3a, b). The unweighted unifrac approach revealed that co-inoculation impacted the microbial community structure in the rhizosphere of turmeric. In the phylogenetic tree, treatment 2 (ZSB2 + AM) was clustered into a separate distinct branch and it indicated highly distant communities compared to the other treatments. Among the samples analyzed, treatment 4 (ZnSO4) and 5 (Absolute control) were clustered together, while treatment 1 (Zinc phosphate + ZSB2) and treatment 3 (Zinc phosphate + AM) were grouped separately (Fig. 3).
Beta diversity analysis of unweighted uniFrac cluster tree showing the dissimilarities among all sample types of turmeric rhizosphere (Treatment 1: Zinc phosphate + ZSB2; Treatment 2: Zinc phosphate + ZSB2 + AM; Treatment 3: Zinc phosphate + AM; Treatment 4: Zinc sulphate; Treatment 5: Absolute control)
The identified OTUs were then classified into 9 phyla (Fig. 4). The maximum number of bacterial reads was found for Chloroflexi followed by Proteobacteria, Actinobacteria and Acidobacteria which accounted for 80% of all samples. The composition of bacteria at the level of phyla were analyzed and showed marked differences between treatments. Co-inoculation of AM with ZSB2 (treatment 2) increased the relative abundances of Choroflexi (54.2%), followed by treatment 1 (Zinc phosphate + ZSB2) with 49.9% and treatment 3 (Zinc phosphate + AM) with 38.3%. Subsequently, major abundance of Acitnobacteria (29.7%) was found in treatment 3 (Zinc phosphate + AM), followed by 18.9% in treatment 2 (ZSB2 and AM) and 18.3% in treatment 1 (Zinc phosphate + ZSB2). Third major phylum was Proteobacteria with 17.1% in treatment 3 (Zinc phosphate + AM), followed by 15.6% in treatment 1 (Zinc phosphate + ZSB2) and by 13.6% in treatment 2 (ZSB2 and AM).
Heat map of the composition of bacterial communities reflected the similarities as well as the differences in the community composition among the treatments (Fig. S4). Acidothermus, Gemmatimonas and Candidatus were the more abundant and Legionella, Reyranella, Ktedonobacter, Saccharopolyspora, Gemmatiorosa, Rhodanobacter, Ludenannella, Actinophytocota and Bellilinea are least abundant microbial communities in all the samples. The population of Conexibacter, Aneromyxobacter, Acidobacteria, Haliangium and Streptomyces was meagre in ZSB2 and AM treated samples. Whereas Streptomyces population was abundant in treatment 3 (Zinc phosphate + AM) when compared to other samples,in the treatment 4 (zinc sulphate), Bdellovibrio, Nocardioides and Sphingomonas were less abundant than the absolute control.
The LefSe (Linear discriminant analysis effect size) revealed that there were more taxa with larger effect sizes (LDA score > 3.0) associated with the treatment of ZSB2 and AM compared to the control sample (Fig. 5). Significant taxa in the ZSB2 and AM treated samples were Bradyrhizobium, Candidatus solibacter, Pedomicrbium, Thermosporothrix, Acinetobacter and Nitrospira. While control samples with the LDA score > − 3.0 and it contained Canidatus nitrosphaera, Herpetosiphon, Bacillus and Gemmatimonas.
Discussion
Co-inoculation of AM and ZSB2 significantly increased plant height and dry weight, respectively, 92.8 and 87.5% over the control. ZSB2 (B. megaterium) has been reported to improve plant growth in different ways like providing beneficial compounds to the host plant and facilitating the uptake of nutrients from the soil environment. Besides Zn solubilization, the organic acids secreted by ZSB2 will also be helpful in the solubilisation of insoluble P compounds [24]. Co-inoculation significantly increased the growth of turmeric (LSD 0.05P = 13.8) and this is expected owing to the synergistic interaction between AM and ZSB2 in promoting plant growth compared to single inoculation with either of them. This can be ascribed to improved nutrient uptake through co-inoculation compared to inoculation with either AM or ZSB2. This supported the observation that co-inoculation of AM and Curtobacterium citreum notably enhanced plant growth and dry weight of Tetradymia comosa [25]. Also, the results clearly indicated the ecological compatibilities between AM and ZSB2, which is in line with earlier reports on the potential of AM + Bacillus spp. association in improving plant growth [26,27,28,29,30]. Most of the Bacillus species directly boost the plant growth either through production of plant growth hormones, acquirement of nutrients and activation of host defense mechanisms or through their synergetic interaction with AM.
During the AM symbiosis, hyphae grow within the root cortex, eventually penetrate the root cells and produce highly branched hyphal tree-like structures called arbuscules which are main structures for nutrient exchanges between the host and fungi [31]. In the course of root colonization, AM increases the growth and yield of crop plants by providing nutrition to host and also imparting other benefits including enhanced photosynthesis, accumulation of secondary metabolites, and improved resistance against biotic and abiotic stresses [32, 33]. Our finding is consistent with the report that co-inoculation of AM and Curtobacterium citreum enhanced growth and dry weight of Tetradymia comosa [24]. Likewise, dual inoculation with Bacillus strains and AM improved the artemisinin content in the Artemisia annua [28], increased the growth and yield of Medicago sativa [25] and Capsicum annuum [34].
Enhanced DH activity due to co-inoculation of AM and ZSB2 suggested a strong influence on the quantitative changes in microbial population as well as soil microbial activity [35]. This corroborated with earlier reports that combined effects of the inoculation with rhizobacteria and AM increased the activity of DH [28, 36, 37]. Enhanced DH activity (LSD 0.05P = 0.15) and therefore microbial activity (LSD 0.05P = 2.62) was further reflected by soil respiration, which was higher in the co-inoculation treatment. This is not surprising since AM and their extraradical hyphae comprise 20–30% of total soil microbial biomass and their contribution to soil respiration is as much as 6–25% [38].
Bacterial community analysis indicated marked shifts in the bacterial community composition with the ZSB2 treatment recording maximum richness and diversity, while the treatment with ZSB2 and AM registered the lowest. However, the observed loss of certain bacterial species in ZSB2 + AM treatment may not change the functioning of the system because of the bacterial redundancy, since different bacterial species may carry out the same functions [39]. Similar results were reported by Akyol et al. [40], who observed that bacterial families such as Acetobacteraceae, Methylobacteriaceae, Alicyclobacillaceae and Armatimonadaceae were reduced consistently by the inoculation of AM, perhaps due to changes in the host plant status caused by the inoculum. Since AM are naturally associated with diverse bacteria, the inoculation of AM may also bring associated bacteria which may lead to changes in bacterial composition. It was also proposed that AM will promote or repress the recruitment of certain bacterial groups in the rhizosphere, regardless of the host species [41] by competing for nutrients with bacteria, and exuding stimulatory or inhibitory compounds [42]. Especially low-abundant bacterial species can have a larger impact on certain ecosystem processes, mainly on soil organic matter decomposition and increase the community resistance to invasion of pathogen, thereby enhancing plant health [43]. On the contrary, we also observed that the sole application of ZSB2 did not affect the soil bacterial diversity and it is buffered due its ability to solubilize certain chemicals which act as nutrients source to resident microbial populations [44].
AM interacts with a wide range of microorganisms in the root and in the rhizosphere. One of the major factors regulating rhizosphere microbiome is the interactions between introduced bacteria and mycorrhizal fungi, which play a crucial role in shaping the microbiome community. Host plant features also mainly influence the extent to which microbes colonize the roots through the production of root exudates. Certain metabolites and hormones produced by plants induce optimistic chemotaxis and assist to recruit a specific group of microbiome near the root zone [14]. At phylum level, Chloroflexi, Actinobacteria and Proteobacteria were considerably abundant due to co-inoculation of ZSB2 and AM. Beneficial interaction of bacterial communities such as Chloroflexi, Acidothermus and Gemmatimonas with AM was documented by Liu et al. [16]. Moreover, this Chloroflexi group of bacteria are known to be competent in exploiting more oxidized forms of C. Hence, their abundance might alter the rhizodeposition and shift in C storage and sequestration. Abundance of such bacterial groups will positively affect nutrient cycling and subsequently plant growth [45]. In addition to that, most of the studies have revealed that Chloroflexi was one of the several most abundant groups associated with the degradation of chlorinated compounds [46].
Sole application of AM increased relative abundances of Actinobacteria taxa and it may be considered as a potential biomarker for improvement in soil nutrient content and plant growth. We also found other bacterial phyla, such as Proteobacteria and Acidobacteria as the most prevailing communities in the rhizosphere of turmeric. The ability to cope with different environmental conditions might also make both phyla to be largely abundant [47,48,49,50]. Mostly, both Actinobacteria and Acidobacteria are abundant in disease-suppressive soils. Besides, these taxa are responsible for exclusion of disease-causing microorganisms [51]. Interestingly, the most abundant genus Acidothermus under phylum Actinobacteria is a unispecific genus described as thermophilic, acidophilic and cellulolytic bacterium which can also degrade chitin [52].
The AM associated bacteria which belonged to the groups of Bradyrhizobium, Candidatus solibacter, Thermosporothrix, Acinetobacter and Nitrospira were recorded by Linear discriminant analysis, besides these bacteria contribute to the establishment and function of the introduced AM fungi. Qin et al. [45] reported that Rhizobiales, Actinomycetales, Sphingobacteriales, Bacillales and Xanthomonadales are abundant bacterial groups associated with AM inoculated soils. During soil C transformation, the interactions between Bradyrhizobium and AM was shown to strongly manipulate soil organic matter turnover, plant growth promotion and nitrogen fixation [53]. Another major group we found is Candidatus solibacter, which participates in nitrate and nitrite reduction and is known for producing enzymes to break down organic carbon available in its environment. This organism is also a recognized biofilm producer and acts as an ecosystem engineer in the soil by enabling this species to adhere to its milieu while also reducing moisture and nutrient fluctuations in the soil under unfavourable environmental conditions [54]. Thermosporothrix bacteria, which belongs to the phylum Chloroflexi which is phylogenetically diverse and express a broad range of metabolic capabilities was also found in the study.
Conclusion
Co-inoculation of AM and ZSB2 was positively related to turmeric growth and soil microbial activity. The bacterial community composition varied distinctly between AM-inoculated and non-inoculated soil samples. Major bacterial phyla found were Chloroflexi, Actinobacteria, Acidobacteria and Proteobacteria in all the samples. Chloroflexi was the more prevailing followed by Actinobacteria in the AM + ZSB2 co-inoculated treatment. From the results, it is apparent that the application of bio-inoculants could significantly change the composition, richness and structure of microbial communities, which might improve and alter the soil functioning. In sustainable agricultural cropping systems, they are likely to enhance the biological processes to maintain soil fertility, plant development and productivity. Nevertheless, further studies are needed to unravel the mechanisms involved in the interaction between the ZSB2 and AM fungi including the functional changes relevant to soil microbial communities.
References
Aarthi S, Suresh J, Leela NK, Prasath D (2020) Multi environment testing reveals genotype-environment interaction for curcuminoids in turmeric (Curcuma longa L.). Ind Crops Prod 145:112090. https://doi.org/10.1016/j.indcrop.2020.112090
Trabelsi D, Mhamdi R (2013) Microbial inoculants and their impact on soil microbial communities: a review. Biomed Res Int. https://doi.org/10.1155/2013/863240
Raklami A, Bechtaoui N, Tahiri A, Anli M, Meddich A, Oufdou K (2019) Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol 10:1106. https://doi.org/10.3389/fmicb.2019.01106
Wang YY, Yin QS, Qu Y, Li GZ, Hao L (2018) Arbuscular mycorrhiza-mediated resistance in tomato against Cladosporium fulvum induced mould disease. J Phytopathol 166:67–74. https://doi.org/10.1111/jph.12662
Rivero J, Álvarez D, Flors V, Azcón-Aguilar C, Pozo MJ (2018) Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol 220:1322–1336. https://doi.org/10.1111/nph.15295
Shi Z, Wang F, Liu Y (2012) Response of soil respiration under different mycorrhizal strategies to precipitation and temperature. J Soil Sci Plant Nutr 12(3):411–420. https://doi.org/10.4067/S0718-95162012005000003
Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M, De Pascale S, Bonini P, Colla G (2015) Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci Hortic 196:91–108. https://doi.org/10.1016/j.scienta.2015.09.002
Sarathambal C, Ilamurugu K, Balachandar D, Chinnadurai C, Gharde Y (2015) Characterization and crop production efficiency of diazotrophic isolates from the rhizosphere of semi-arid tropical grasses of India. Appl Soil Ecol 87:1–10. https://doi.org/10.1016/j.apsoil.2014.11.004
Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Iqbal MI, Mohammad O (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41. https://doi.org/10.1016/j.micres.2015.11.007
Turrini A, Avio L, Giovannetti M, Agnolucci M (2018) Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: the challenge of translational research. Front Plant Sci 9:1407. https://doi.org/10.3389/fpls.2018.01407
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778. https://doi.org/10.1093/jxb/eri197
OrdoñeznYM FBR, Lara LR, Rodriguez A, Uribe- Vélez D, Sanders IR (2016) Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. PLoS ONE 11:e0154438. https://doi.org/10.1371/journal.pone.0154438
Song X, Liu M, Wu D, Griffiths B, Jiao J, Li H, Hu F (2015) Interaction matters: synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl Soil Ecol 89:25–34. https://doi.org/10.1016/j.apsoil.2015.01.005
Sarathambal C, Khankhane PJ, Gharde Y, Kumar B, Varun M, Arun S (2017) The effect of Plant growth promoting rhizobacteria on the growth, physiology, and Cd uptake of Arundo donax L. Int J Phytoremediation 19(4):360–370. https://doi.org/10.1080/15226514.2016.1225289
Dinesh R, Srinivasan V, Hamza S, Sarathambal C, Anke Gowda SJ, Ganeshamurthy AN, Gupta SB, Aparna Nair V, Subila KP, Lijina A, Divya VC (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186. https://doi.org/10.1016/j.geoderma.2018.02.013
Liu Y, Sun Q, Li J, Lian B (2018) Bacterial diversity among the fruit bodies of ectomycorrhizal and saprophytic fungi and their corresponding hyphosphere soils. Sci Rep 8:11672. https://doi.org/10.1038/s41598-018-30120-6
Casida LE Jr, Klein DA, Santoro R (1964) Soil dehydrogenase activity. Soil Sci 98:371–378. https://doi.org/10.1097/00010694-196412000-00004
Phillips JM, Hayman DS (1970) Improved produces for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Bukin Y, Galachyants Y, Morozov I (2019) The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci Data 6:190007. https://doi.org/10.1038/sdata.2019.7
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335. https://doi.org/10.1038/nmeth.f.303
Rognes T, Flouri T, Nichols B, Quince C, Mahe F (2016) V search a versatile open source tool for metagenomics. Peer J. https://doi.org/10.7717/peerj.2584
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Dinesh R, Anandaraj A, Kumar A, Bini YK, Subila KP, Aravind R (2015) Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol Res 173:34–43. https://doi.org/10.1016/j.micres.2015.01.014
Bourles A, Guentas L, Charvis C (2020) Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza 30:121–131. https://doi.org/10.1007/s00572-019-00929-8
Medina A, Probanza A, Gutierrez Mañero FJ, Azcón R (2003) Interactions of arbuscular-mycorrhizal fungi and Bacillus strains and their effects on plant growth, microbial rhizosphere activity (thymidine and leucine incorporation) and fungal biomass (ergosterol and chitin). Appl Soil Ecol 22:15–28. https://doi.org/10.1186/s40694-019-0086-5
Adriana MA, Rosario A, Juan RL, Ricardo A (2007) Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus coinoculated: physiologic and biochemical traits. J Plant Growth Regul 7:10–18. https://doi.org/10.1007/s00344-007-9024-5
Flores AC, Luna AAE, Portugal OP (2007) Yield and quality enhancement of marigold flowers by inoculation with Bacillus subtilis and Glomus fasciculatum. J Sustain Agr 31:21–31. https://doi.org/10.1300/J064v31n01_04
Awasthi A, Bharti N, Nair P, Singh R, Shukla A, Gupta M, Darokar M, Kalra A (2011) Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisin content in Artemisia annua L. Appl Soil Ecol 49:125–130. https://doi.org/10.1016/j.apsoil.2011.06.005
Alam M, Khaliq A, Sattar A, Shukla RS, Anwar M, Seema Dharni S (2011) Synergistic effect of arbuscular mycorrhizal fungi and Bacillus subtilis on the biomass and essential oil yield of rose-scented geranium (Pelargonium graveolens). Arch Agron Soil Sci 57:889–898. https://doi.org/10.1080/03650340.2010.498013
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GED, Eastmond PJ (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356:1175–1178. https://doi.org/10.1126/science.aam9970
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashra M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448
Shinde SK, Shinde BP, Patale SW (2013) The alleviation of salt stress by the activity of AM fungi in growth and productivity of onion (Allium cepa l.) plant. Int J Life Sci Pharma Res 3:11–15
Thilagar G, Bagyaraj DJ, Rao MS (2016) Selected microbial consortia developed for chilly reduces application of chemical fertilizers by 50% under field conditions. Sci Hortic 198:27–35. https://doi.org/10.1016/j.scienta.2015.11.021
Romero E, Fernandez-Bayo J, Diaz J, Nogales R (2010) Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Appl Soil Ecol 44:198–204. https://doi.org/10.1016/j.apsoil.2009.12.006
Mengual C, Mauricio S, Azcón R, Roldán A (2014) Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions. J Environ Manage 134:1–7. https://doi.org/10.1016/j.jenvman.2014.01.008
Xun F, Xie B, Liu S, Guo C (2015) Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ Sci Pollut Res Int 22(1):598–608. https://doi.org/10.1007/s11356-014-3396-4
Moyano FE, Kutsch WL, Schulze ED (2007) Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biol Biochem 39:843–853. https://doi.org/10.1016/j.soilbio.2006.10.001
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54(4):655–670. https://doi.org/10.1046/j.1351-0754.2003.0556.x
Akyol TY, Niwa R, Hirakawa H, Maruyama H, Sato T, Suzuki T, Fukunaga A, Sato T, Yoshida S, Tawaraya K, Saito M, Ezawa T, Sato S (2019) Impact of introduction of arbuscular mycorrhizal fungi on the root microbial community in agricultural fields. Microbes Environ 34(1):23–32. https://doi.org/10.1264/jsme2.ME18109
Selvakumar G, Krishnamoorthy R, Kim K, Sa TM (2016) Genetic diversity and association characters of bacteria isolated from arbuscular mycorrhizal fungal spore walls. PLoS ONE 11:e0160356. https://doi.org/10.1371/journal.pone.0160356
Tsavkelova EA, Cherdyntseva TA, Netrusov AI (2005) Auxin production by bacteria associated with orchid roots. Microbiol 74:46–53. https://doi.org/10.1007/s11021-005-0027-6
Kurm V, van der Putten WH, Pineda A, Hol WHG (2018) Soil microbial species loss affects plant biomass and survival of an introduced bacterial strain, but not inducible plant defences. Ann Bot 121(2):311–319. https://doi.org/10.1093/aob/mcx162
Doongar RC, Rathore AP, Sharma S (2020) Effect of halotolerant plant growth promoting rhizobacteria inoculation on soil microbial community structure and nutrients. Appl Soil Ecol 150:103461. https://doi.org/10.1016/j.apsoil.2019.103461
Qin H, Brookes PC, Xu J (2016) Arbuscular mycorrhizal fungal hyphae alter soil bacterial community and enhance polychlorinated biphenyls dissipation. Front Microbiol 7:939. https://doi.org/10.3389/fmicb.2016.00939
Vik U, Logares R, Rakel B, Rune H, Carlsen T, Ingrid B, Anne-Brit K, Oksta OA, Kauserud H (2013) Different bacterial communities in ectomycorrhizae and surrounding soil. Sci Rep 3:3471. https://doi.org/10.1038/srep03471
Ofek-Lalzar M, Sela N, Goldman-Voronov M (2014) Niche and host-associated functional signatures of the root surface microbiome. Nat Commun 5:4950. https://doi.org/10.1038/ncomms5950
Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587. https://doi.org/10.1038/ismej.2014.17
Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M (2015) Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol 17:610–621. https://doi.org/10.1111/1462-2920.12452
Hamonts K, Trivedi P, Garg A, Janitz C, Grinyer J, Holford P, Botha FC, Anderson IC, Singh BK (2018) Field study reveals core plant microbiota and relative importance of their drivers. Environ Microbiol 20(1):124–140. https://doi.org/10.1111/1462-2920.14031
Bhattacharyya D, Duta S, Sang-Mi Yu, Jeong SC, Lee YH (2018) Taxonomic and functional changes of bacterial communities in the rhizosphere of kimchi cabbage after seed bacterization with Proteus vulgaris JBLS202. Plant Pathol J 34(4):286–296. https://doi.org/10.5423/PPJ.OA.03.2018.0047
Barabote RD, Xie G, Leu DH, Normand P, Necsulea A, Daubin V (2009) Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus provides insights into its ecophysiological and evolutionary adaptations. Genome Res 19:1033–1043. https://doi.org/10.1101/gr.084848.108
Xu J, Feng Y, Wang Y, Luo X, Tang J, Lin X (2015) The foliar spray of Rhodopseudomonas palustris grown under stevia residue extract promotes plant growth via changing soil microbial community. J Soil Sediments 65:180–192. https://doi.org/10.1007/s11368-015-1269-1
Pearce DA, Newsham KK, Thorne MAS, Clavo-Bado L, Kresk M, Laskaris P (2012) Metagenomic analysis of a southern mairitime antartic soil. Front Microbiol 3:403. https://doi.org/10.3389/fmicb.2012.00403
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: CS, RD VS and TES. Soil physico-chemical properties and soil microbial activity: VS RD, CS and VJ. Bacterial community analysis: CS, RD TES and MM. CS wrote the draft of the manuscript; RD revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarathambal, C., Dinesh, R., Srinivasan, V. et al. Changes in Bacterial Diversity and Composition in Response to Co-inoculation of Arbuscular Mycorrhizae and Zinc-Solubilizing Bacteria in Turmeric Rhizosphere. Curr Microbiol 79, 4 (2022). https://doi.org/10.1007/s00284-021-02682-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-021-02682-8