Abstract
This study explored the differences in the microbial diversity and physicochemical properties of mushroom residue and cow manure to provide a theoretical basis for the use of mushroom residue as cow bedding. High-throughput sequencing was used to analyze the bacterial community composition of mushroom residue and cow manure bedding and determine the physical and chemical properties of these different bedding materials. The results showed that the bacterial communities in the two types of bedding materials could be categorized into 6 classes, 13 orders, 32 families, and 48 genera. The dominant genus in the mushroom residue bedding samples after use by cows was Lactobacillus (36.37%) followed by Corynebacterium (22.15%). The dominant group in the cow manure bedding samples after use was “other” (28.8%), followed by Solibacillus (8.76%). The different bedding materials contained varying number of bacterial species. After use, 499 bacterial species were present in the cow manure bedding, while only 345 bacterial species were present in the mushroom residue bedding. The utilization rate of the mushroom residue bedding by dairy cows was 79%, whereas that of the cow manure bedding was 61%. The results of this study provide a theoretical basis for the application of mushroom residue bedding for dairy cows.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The edible mushroom industry, which is an important part of modern agriculture, is the second largest vegetable industry in China. It is characterized by diverse and extensive cultivation materials and conditions [1]. The mushroom industry is developing rapidly in the Yellow River Delta and other areas that are not suitable for traditional crop cultivation. According to the statistics of the China Edible Fungus Association, the total output of edible fungi in China in 2017 was 37.12 million tons, 3.21% higher than the output in 2016 (35.97 million tons). At present, China is the largest producer of edible mushrooms worldwide. Consequently, it is also the largest producer of edible mushroom residue. Edible fungus residue is the residual waste (the culture medium) after the product has been harvested. Edible fungus residue is rich in protein, polysaccharides, mycelium, and other nutrients [2, 3]; however, this nutrient-rich and productive resource is often overlooked. Based on an average edible fungus biological efficiency of 40% and the total edible mushroom residue output of approximately 14.85 million tons in China in 2017, the mushroom residue output is enormous [4]. In recent years, several studies have explored the best use of mushroom residue by recycling it within the mushroom industry. Secondary planting of mushrooms is a common way to reuse mushroom residue. This strategy has been applied to the cultivation of Pleurotus ostreatus [5], Volvariella volvacea [6], Agaricus bisporus [7], Agaricus blazei [8], among others, leading to significant economic benefits. Mushroom residue has also been used as a substrate for saccharification and fermentation with the addition of cellulase and Saccharomyces cerevisiae AM12. The ethanol extraction rate of this process is 77% [9]. Alternatively, Hu et al. [10] used compost residue to fertilize rice, which increased the yield by 20.55% compared with conventional fertilization. Similarly, Sheng et al. [11] raised Qingshan sheep using Flammulina velutipes dregs instead of bran in their diet, and their weight increased by 16.58%, thereby increasing the economic value by 29.72% compared with sheep raised on conventional feed.
The Yellow River Delta is located at the junction of the Yellow Sea and the Bohai Sea. The area is covered by both the national development plan of the Yellow River Delta High Efficiency Ecological Economic Zone and the development plan of the Blue Economic Zone of Shandong Peninsula. Owing to its unique mud flat characteristics, climate conditions, and soil characteristics, the Yellow River Delta has a wide distribution of salt and alkali zones. Its unique soil environment has caused many problems for the agricultural industrialization of the region, including difficulties in the normal growth of ordinary crops, recycling agricultural wastes, and the integration of various industries, which have restricted the development of local industries [12].
Owing to the rich forage grass and well-developed dairy cattle breeding industry in the Yellow River Delta, the idea of using dried edible fungus residue as dairy cattle bedding was conceived and exploratory experiments were conducted on small dairy farms. Using sensory indicators, it was found that when dried edible fungus residue was used as dairy cattle bedding, the utilization rate of the bedding was higher than that of cow manure, the conventional bedding material of the region. The cleanliness of the dairy cow shelter environment is a key factor in ensuring the health of dairy cows and improving their milking capacity. Dairy cows spend 50% to 60% of their day resting on their stomach; therefore, the materials and comfort of their bedding are important for their health and milking capacity [13]. A poor bedding environment increases the risk of lameness, foot-and-toe disease, and mastitis in dairy cows. Currently, cow manure [14] and sandy soil (after solid–liquid separation) are widely used as bedding materials for dairy cows. However, manufacture of sandy soil bedding is labor intensive, and the fecal waste recovery equipment can easily become blocked. In contrast, the microbial content of cow manure bedding is high, and it can easily cause mastitis in dairy cows [15].
In the present study, we aimed to promote the development and utilization of saline–alkali land in the Yellow River Delta; reduce the waste of mushroom residue resources; develop new dairy bedding; develop a symbiosis model of modern agriculture, ecology, and an edible fungus/animal husbandry/recycling industry; and promote the development of a green recycling industry in the region. To achieve this, we (i) determined the physicochemical properties of dry mushroom residue and solid–liquid separated cow manure, (ii) observed the behavior of dairy cows, and (iii) determined the microbial community of dry mushroom residue and solid–liquid separated cow manure. This combined information provides a theoretical basis for the development and utilization of dried mushroom residue as bedding material for dairy cows.
Materials and Methods
Cattle Farm Selection and Treatment of Cow Bedding
The experiment was conducted on the Dongying Dadi dairy cattle farm. The cow manure material used was fermented cow manure from the Dongying Dadi dairy farm after solid–liquid separation, while the mushroom residue material used was industrial mushroom residue from the Shandong Huaao group (Dongying City, China) after crushing and airing. An internationally advanced four-row, free and scattered feeding mode was used in the cowsheds. The cowsheds were equipped with automatic fecal cleaning and automatic fecal sewage treatment via a scraping board. Intelligent equipment providing feed with total mixed rations was used.
Experimental Design
Forty cows with the same number of births and similar physical conditions were selected and split into two groups of 20 cows each, with each group kept in a separate enclosure. The feed, feeding management, and milking times of the two groups were the same. Only one bedding material, either cow manure after solid–liquid separation or dried mushroom residue, was provided in each enclosure. After solid–liquid separation, the cattle manure or dried mushroom residue was laid out to a height of 20 cm. Far-infrared, high-definition digital cameras were installed in each enclosure facing the beds, and the use of the bedding material by the cows was recorded 24 h a day for 1 month.
Behavioral Observations
Using a continuous recording method, the bedding utilization rate [cows using bedding/(total relaxing cows − feeding cows)] and the comfort index of the cows (cows using bedding/total relaxing cows) were recorded, and the resting behavior and morphology of the cows were observed continuously.
Collection of Dairy Cow Bedding Samples
Samples of the cow manure material were collected from the solid–liquid separation and post-treatment workshop of the Dongying Dadi dairy farm. Samples were also collected from the fermentation workshop after solid–liquid separation and post-treatment and a normal cow shed. Industrial Hypsizygus marmoreus mushroom residue samples were collected from the Shandong Huaao group after crushing and airing and from the mushroom residue bedding after the cows had used it for 1 month. Three sampling points were randomly selected in each workshop and cowshed to collect bedding samples. The five bedding material groups were named as follows: nainiu, cow manure after use by cows; gu-ye, unfermented cow manure after solid–liquid separation; fajiao, fermented cow manure after solid–liquid separation; junzha, mushroom residue; and diwuzu, mushroom residue after use by dairy cows. The collected samples were further separated into “used” and “unused” bedding before analysis to ensure appropriate representation of all results.
Determination of Physical Properties of Dairy Cow Bedding Samples
The volumetric weight, water-holding capacity, total porosity, aeration porosity, water-holding porosity, gas–water ratio, water/steam ratio, and void ratio of the bedding materials were measured using the ring knife method [16]. The pH of the materials was measured via water extraction (water: bedding material = 10: 1).
DNA Extraction, Sequencing, and Bioinformatics
The collected bedding material samples were sent to the Shanghai Meiji Biomedical Technology Co., Ltd. First, total DNA extraction was performed according to the instructions of the E. Z. N. A.® soil kit (Omega Bio-Tek, Norcross, GA, USA). A NanoDrop2000 system was used for DNA concentration and purity detection. PCR amplification of the V3–V4 variable region was performed using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGTWTCTAAT-3′). The amplification procedure was: pre-denaturation at 95 °C for 3 min; followed by 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s; and finally, extension at 72 °C for 10 min (PCR instrument: ABI GeneAmp®, type 9700). The amplification system was 20 µL:4 µL 5 × FastPfu buffer, 2 µL 2.5 mM dNTPs, 0.8 µL 5 µM primer, 0.4 µL FastPfu polymerase, and 10 ng DNA template.
The PCR products were recovered using a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), tris–HCl elution, and 2% agarose electrophoresis. QuantiFluor™-ST (Promega, USA) was used for quantitative detection. A 2 × 300 bp PE library was constructed using the purified amplified fragments according to the standard operating procedures of the Illumina MiSeq platform (Illumina, San Diego, USA). The steps used for constructing the library were as follows: (1) The “Y”-shaped connector was connected; (2) magnetic beads were used to screen and remove the self-connecting segment of the joint; (3) PCR amplification was used for the enrichment of library templates; and (4) sodium hydroxide was used to denature the samples and produce single-strand DNA fragments. Then, 30 mL of the prepared PCR product for each sample was used for high-throughput sequencing using the Illumina MiSeq platform.
Data processing and biodiversity analyses were conducted on the I-sanger biological information analysis cloud platform (http://www.i-sanger.com/). The paired-end reads obtained from MiSeq sequencing were merged; then, the reads were filtered based on quality. After the samples were differentiated, operational taxonomic unit (OTU) cluster analysis and species taxonomic analyses were conducted.
The Usearch platform (version 7.0 http://drive5.com/usearch/) was used for the following: to extract non-repetitive sequences from the optimized sequences (this is convenient to reduce the amount of redundant calculations in the analysis process) (http://drive5.com/usearch/manual/dereplication.html); to remove any single sequences without repetition (http://drive5.com/usearch/manual/singletons.html); and to cluster any non-repetitive sequences (excluding single sequences) according to 97% similarity. Chimeras were removed in the clustering process, and representative OTU sequences were obtained. In order to obtain species classification information corresponding to each OTU, the RDP classifier Bayesian algorithm was used to analyze the 97% OTU representative sequences at similar levels and compare them to the Silva database (Release128 http://www.arb-silva.de) at each taxonomic level (domain, kingdom, phylum, class, order, family, genus, and species).
Statistical Analysis
Statistical analyses of the community composition of each sample were conducted. Based on the OTU analysis results, multiple diversity index analyses were conducted for each OTU; sequencing depth was also detected. Based on the classification results, multiple diversity index analyses were conducted for each OTU. The taxonomic information was used for the statistical analysis of community structure at each classification level.
Results were analyzed using a t test and principal correlation analysis (PCoA). In all analyses, a probability value less than 0.05 was considered statistically significant (P < 0.01).
Results and Analyses
Cow Behavior Data Collection
The bedding utilization rate and comfort index of the dairy cows differed significantly between bedding types at the 5% and 1% levels. The bedding utilization rate of the mushroom residue was 79%, which was 18% higher than that of the cow manure (see Table 1; Supplementary Figures 1 and 2).
Physical and Chemical Properties of the Dairy Cow Bedding Samples
All cattle manure and mushroom residue samples were acidic (see Table 2). The water content of the mushroom residue was only 14.352%. The aeration porosity of the cattle manure was low, making it easy for anaerobic bacteria to grow. With regard to matrix utilization, the pore size ratio of the mushroom residue samples was about 1:5.1. The pore size ratio of the cow manure samples was about 1:19.7. Table 3 outlines the results of the dairy cow bedding sample volumetric weight, total porosity, aeration porosity, water-holding porosity, water/steam ratio, and void ratio.
Diversity Analysis
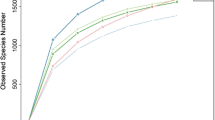
The order of species richness (ACE index) of the samples was gu-ye > nainiu > fajiao > diwuzu > junzha (Fig. 1). There were significant differences in diversity among the five groups, specifically between gu-ye and junzha. The rarefaction curves tended to be flat (Supplementary Figure 3), indicating that the number of samples was reasonable and that the results reliably reflect the bacterial communities of the different bedding materials [17].
ACE index (for species diversity) of different dairy cow bedding materials. *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01; and ***P ≤ 0.001. Nainiu: cow manure after use by cows; gu-ye: unfermented cow manure after solid–liquid separation; fajiao: fermented cow manure after solid–liquid separation; junzha: mushroom residue; diwuzu: mushroom residue after use by dairy cows
Community Composition of Different Bedding Materials Before and After Use by Cows
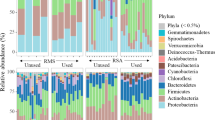
Five bacterial phyla (Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes, and Chloroflexi), 6 classes, 13 orders, 32 families, and 48 genera were detected in the bedding samples. At the genus level, the dominant bacterial group in the unused dairy cow bedding was “other” (unknown genus), with a relative abundance of 66.02%, followed by Pseudomonas (31.53%), Serratia (30.64%), and Weissella (13.98%) (Table 4). The dominant bacterial group of the dairy cow bedding after use was Lactobacillus, with a relative abundance of 36.40%, followed by “other” (35.68%), Corynebacterium (26.61%), and Lysinibacillus (12.39%) (Table 5).
Community Composition of Different Bedding Materials
Alpha Diversity
Figure 2 (following Wang et al. [18]) shows the bacterial abundance (at the genus level) of the five bedding materials. There were obvious differences in the relative abundances of the bacterial groups among the different bedding materials. The dominant genus in the mushroom residue (junzha) was Serratia, with a relative abundance of 30.63%, followed by Pseudomonas (24.92%), Weissella (13.97%), Enterobacter (10.75%), and Pantoea (8.05%). In contrast, the dominant genus in the mushroom residue used by the dairy cows (diwuzu) was Lactobacillus, with a relative abundance of 36.37%, followed by Corynebacterium (22.15%), Lysinibacillus (7.88%), Pediococcus (7.07%), and “other” (6.88%). The dominant bacterial group in the unfermented cow manure after solid–liquid separation (gu-ye) was “other,” with a relative abundance of 34.31%, followed by Corynebacterium (7.09%), Aerococcaceae (4.67%), Romboutsia (4.3%), and Brachybacterium (4.03%). The dominant genus in the cow manure (fajiao) was “other,” with a relative abundance of 25.94%, followed by Pseudoxanthomonas (9.09%), Planifilum (9.01%), Bacillus (7.45%), Pseudomonas (6.19%), and Acinetobacter (5.9%). In contrast, the dominant genus in the cow manure after use by the cows (nainiu) was “other,” with a relative abundance of 28.8%, followed by Solibacillus (8.76%), Glutamicibacter (6.35%), Brachybacterium (6.17%), Bacillus (5.63%), and Flavobacterium (5.11%).
Composition of bacterial communities in different dairy cow bedding materials. Each type of bedding material was sampled and analyzed in triplicate. 1: Cow manure after use by cows; 2: unfermented cow manure after solid–liquid separation; 3: fermented cow manure after solid–liquid separation; 4: mushroom residue; 5: mushroom residue after use by dairy cows
Beta Diversity
The number of bacterial species in the bedding materials followed the order: gu-ye > nainiu > fajiao > diwuzu > junzha (Fig. 3). The cow manure contained fewer species after it had been used by the cows than after solid–liquid separation. The fermented cow manure after solid–liquid separation had 219 more species of bacteria than the mushroom residue. After both types of bedding had been used by the cows, the fermented cow manure contained 115 more species of bacteria than the mushroom residue, and the difference was statistically significant.
Diagrams showing the number of bacterial species in different dairy cow bedding materials. a Venn diagram showing the number of species that overlap in the five types of bedding materials. b Bar graph showing the total number of species for each of the bedding types. Nainiu: cow manure after use by cows; gu-ye: unfermented cow manure after solid–liquid separation; fajiao: fermented cow manure after solid–liquid separation; junzha: mushroom residue; diwuzu: mushroom residue after use by dairy cows
Relative Abundances of Common Taxa
The differences in the community compositions of the five bedding groups are shown in the PCoA plot (Fig. 4). Regardless of whether the cow manure bedding was tested after solid–liquid separation, whether it was fermented or unfermented, or whether it had been used by the cows, the samples had highly similar compositions. However, there were large differences in species composition between the mushroom residue samples and the other samples. The 15 most abundant bacterial genera were analyzed further (Fig. 5). There were significant differences in abundance among the five bedding groups for Pseudomonas, Serratia, Lactobacillus, Corynebacterium, Weissella, Enterobacter, Lysinibacillus, Brachybacterium, Isoptericola, Pseudoxanthomonas, Planifilum, Solibacillus, Glutamicibacter, and Pantoea.
Principal correlation analysis (PCoA) using five different dairy cow bedding materials, showing the similarity of bacterial taxa present for each bedding sample along PC1 and PC2. Each of the five bedding types had three samples, which are denoted as individual symbols in the plot. Clustering of points shows that the bacterial compositions were similar for each bedding type, all cow manure bedding had similar bacterial compositions, and mushroom residue samples were different than cow manure samples. Nainiu: cow manure after use by cows; gu-ye: unfermented cow manure after solid–liquid separation; fajiao: fermented cow manure after solid–liquid separation; junzha: mushroom residue; diwuzu: mushroom residue after use by dairy cows
Relative abundances of the 15 most common bacterial genera found in each of the bedding types. Results were obtained via a Kruskal–Wallis H test. Levels of significance are displayed on the right, where * indicates 0.01 < P ≤ 0.05 and ** indicates 0.001 < P ≤ 0.01. Nainiu: cow manure after use by cows; gu-ye: unfermented cow manure after solid–liquid separation; fajiao: fermented cow manure after solid–liquid separation; junzha: mushroom residue; diwuzu: mushroom residue after use by dairy cows
Discussion
This study found that, in cow bedding before and after use by cows, certain differences exist in the microbial communities, both in terms of community structure and species richness (Fig. 2). The unfermented cow manure after solid–liquid separation (gu-ye) had the highest bacterial richness, followed by cow manure after use by cows (nainiu), cow manure (fajiao), and mushroom residue used by dairy cows (diwuzu); the lowest bacterial richness was found in mushroom residue (junzha). Bacterial richness in the mushroom residue bedding was significantly lower than that in the cow manure bedding, regardless of before or after use by cows. According to the PCoA (Fig. 4), the species composition of the mushroom residue differed from that of cow manure. The cow manure had fewer species after it had been used by the cows than after solid–liquid separation. The fermented cow manure contained 219 more species of bacteria after solid–liquid separation than the mushroom residue. After both types of bedding had been used by the cows, the fermented cow manure had 115 more species of bacteria than the mushroom residue. The differences were statistically significant. This indicates that the mushroom residue bedding is better than the cow manure bedding (Fig. 3).
Bedding types differed significantly in the relative abundances of specific bacterial genera. There were significant differences in the relative abundances of Pseudomonas and Serratia (in the top 10 bacterial genera) between bedding materials before and after use. The relative abundance of unknown bacteria in the mushroom residue and mushroom residue used by dairy cows was approximately 6%, whereas in the three types of cow manure samples, they were approximately 30%. The community structure in the three cow manure samples was also relatively complex (Fig. 2). This large number of unknown bacteria could indicate hidden dangers to the health of the dairy cows, increasing their risk of contracting mastitis and other diseases.
The higher the comfort of the bedding, the longer the cows spend lying down, and the better the lactation of dairy cows [19]. The bed-use rate of the mushroom residue was 79%, and the comfort degree of the dairy cows was 76%, which was 20% higher than that of the cow manure, a statistically significant difference. The ventilation porosity of the mushroom residue was 11.112%, whereas the ventilation porosity of the cow manure was 3.089%. This low ventilation porosity of the cow manure encouraged the growth of a large number of anaerobic microorganisms. Conversely, the pore size ratio of the mushroom residue samples was about 1:5.1, allowing it to be used as a substrate for soilless cultivation as crops can grow normally in it without anaerobic restrictions. From the perspective of matrix utilization, a suitable bulk density is 0.1–0.5 g/cm3 [20], total porosity is 60–70%, water-holding capacity is 55–75%, and crops can grow well within the range of 1:1.5–4 [21]. The ratio of the size to porosity of the mushroom residue was 1:5.1, and the ratio of the size to porosity of the cattle manure was 1:5.1. The void ratio was 1:19.7. Therefore, after use as cow bedding, edible fungus residue can be reused for soilless cultivation, facilitating the possible development of an agricultural industry cycle.
Currently, because of transportation costs, the applicability of these results would only be viable in areas with high dairy and edible mushroom farming. In the Yellow River Delta, the dairy farming industry is well developed and the mushroom industry even more so. Currently, the integration of industrial structures in the Yellow River Delta is low; the utilization rate of agricultural waste is equally low. The utilization rate of agricultural waste can be improved using mushroom residue as bedding material for dairy cows, promoting the development and integration of the mushroom and dairy farming industries. Research into the application of mushroom residue as bedding material for dairy cows is still lacking. We have obtained ideal results in this pilot study.
Conclusion
We believe that mushroom residue can be used as a new type of dairy cow bedding material that can be used to reduce the incidence of cow diseases and improve the comfort of cows. It is also more conducive to cow lactation than conventional bedding. This study provides a good theoretical basis for the comprehensive utilization of mushroom residue and the selection of cow bedding materials; additionally, it provides a “recyclable” green model for agricultural production in the Yellow River Delta. However, additional research is required to further support the dairy farming and mushroom industries beyond this relatively small area in order to achieve more efficient and comprehensive utilization of residual products.
Data Availability
Sequencing data can be accessed at the NCBI database under accession number PRJNA698067.
References
Hu Q, Zhang R (2013) Recycling mode of fungi industry can promote efficiently utilization of agricultural wastes. Chin J Agric Resour Reg Plann 34:113–119
Zhang T, Han J, Li J, Xie H, Gong Z (2016) Comprehensive utilization and research status of edible fungi residues. Shandong Agric Sci 48:146–150
Sheng P, Sang Y, Wang J, Zhang G, Liu C, Chen Q (2017) Physical-chemical properties and safety as assessment of industrial mushroom residues. J Beijing Univ Agric 32:42–45
Wei Z, Zhou G, Hu Q (2010) Research and utilization of edible fungi residue. Edible Fungi China 29:3–6
Han J, Sun J, Sun R, Lin F, Wan L, Yang P, Yao Q, Gong Z (2019) Analysis and evaluation on Pleurotus ostreatus cultivation using spent industrialized Flammulina velutipes substrate. Edible Fungi China 38:27–31
Ren H, Ren P, Gong Z, Qu L, Huang C, Guo H, Wan L (2018) Test on Volvaraia volvacea cultivation with spent substrate of industrialized cultivation mushrooms. Edible Med Mushrooms 26:378–380
Ke B, Cai Z, Lu Z, Liao J, Lan Q (2018) Fermentation characteristics of spent mushroom substrate of Pleurotus enryngii and Flammulina velutiper and cultivation test of Agaricus bisporus. Jiangsu Agric Sci 46:153–155
Li B, Chen Z, Ling L (2016) Screening on the formulas of Agaricus blazei cultivated by waste residue. Edible Fungi China 35:31–34
Asakawa A, Sasaki C, Asada C, Nakamura Y (2012) Evaluation of waste mushroom medium as a fermentable substrate and bioethanol production. Int J Modern Phys Conf Ser 6:745–750. https://doi.org/10.1142/S2010194512004084
Hu Q, Wei Z, Wang H (2011) Agaricus bisporus residue compost and its fertilizer efficiency. J Agro-Environ Sci 30:1902–1909
Sheng Q, Gong Z, Tao H (2011) Effect of enoki mushroom residue on mutton sheep fattening. Feed Rev 03:1–3
Liu L, Li Y, Liu Z, Zhao Y, Ren D, Guo Q, Sun S (2019) Construction and demonstration of ecocycle symbiosis model based on saline–alkali land improvement—setting the Yellow River Delta area as an example. Shandong Land Resour 35:59–63
Luo Y, Li X, Ma C, Ma H (2018) Effects of different bedding materials on lying behaviors and health condition of dairy cows. China Dairy Cattle 03:14–17
Huang L, Wang L, Li Y, Guo J, Jin X, Zhang J (2016) Effects of different sleeping materials on the lying behavior of dairy cows. Anim Husb Vet Med 48:67–70
Meng Y (2014) Effects of cow manure bedding on lying behavior of dairy cows and environmental hygiene. Dissertation. Northeast Agricultural University, Harbin
Li Y, Ji Y, Yu P, Wu Z, Liu M (2016) Screening test for mixed soilless culture matrix based on different physical and chemical properties. North Hortic 359:44–48
Perini L, Gostinčar C, Gunde-Cimerman N (2019) Fungal and bacterial diversity of Svalbard subglacial ice. Sci Rep 9:1–15. https://doi.org/10.1038/s41598-019-56290-5
Wang Z, Yang Y, Xia Y, Wu T, Zhu J, Yang J, Li Z (2019) Time-course relationship between environmental factors and microbial diversity in tobacco soil. Sci Rep 9:1. https://doi.org/10.1038/s41598-019-55859-4
Shan C, Guo J, Zhao J, Wang C, Zhao S, Wang Y, Gao Y (2019) Effects of manure/rice husk ratio in bedding on lying behavior, digestive performance and milk performance of dairy cows. Chin J Anim Nutr 31:1645–1654
Suo L (2012) The conversion and application of several agro-forestry biomass residues into growing media, Dissertation. Beijing Forestry University, Beijing
Pu S, Feng G, Li P, Zhang S, Sun X, Ding F (2012) Studies on determination of the physical and chemical characteristics of soilless cultivation substrates and their application. Xinjiang Agric Sci 49:267–272
Funding
This work was supported by the earmarked fund for Modern Agro-Industry Technology Research System (Grant No. CARS-20); the Shandong Upgraded Project of “Bohai Granary” Science and Technology Demonstration Engineering (Grant No. 2019BHLC005); and the Big Science Program of Shandong Province (Grant No. 20190103).
Author information
Authors and Affiliations
Contributions
LJ and RP helped design this study and performed the statistical analysis. LJ carried out the study and collected important background information. LJ, RP, and QL are responsible for the concepts, design, definition of intellectual content, literature search, data acquisition, data analysis, and manuscript preparation. CS, RH, WL, and GH helped with data acquisition, data analysis, and statistical analysis. ZH, GX, and LJ performed the literature search, acquired data, and edited the manuscript. LJ drafted the manuscript. RP and QL reviewed the manuscript. All authors have read and approved the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
The study complied with the animal experiment ethics requirements of the Shandong Academy of Agricultural Sciences. Permission was granted to conduct the study on animals at the Dongying Dadi dairy farm (SAAS-2019-001, 2019.04.18).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2021_2412_MOESM3_ESM.tif
Fig. 3 Rarefaction curves of different dairy cow bedding materials. All three samples of each bedding type are represented. Note: Nainiu: cow manure after use by cows; gu-ye: unfermented cow manure after solid–liquid separation; fajiao: fermented cow manure after solid–liquid separation; junzha: mushroom residue; diwuzu: mushroom residue after use by dairy cows. Supplementary file3 (TIF 852 KB)
284_2021_2412_MOESM4_ESM.tif
Fig. 4 Form confirming that the requirements for animal experiment ethics at Shandong Academy of Agricultural Sciences were met in this study. Supplementary file4 (TIF 582 KB)
Rights and permissions
About this article
Cite this article
Lian, J., Qu, L., Ren, P. et al. Industrial Mushroom Residue as Cow Bedding: Analysis of Microbial Diversity and Applications. Curr Microbiol 78, 1448–1457 (2021). https://doi.org/10.1007/s00284-021-02412-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02412-0