Abstract
Although much is known about the mechanisms affecting cholera spread, cholera outbreaks occur annually in Iran. The aim of this study was to characterize and assess the clonal correlation of strains obtained from an outbreak in 2013 in Iran. Thirty-three strains of Vibrio cholerae were isolated from stool sample of patients majority of them belonged to Afghan nationality. PCR and sequencing analysis was performed to characterize virulence and resistance associates genes and cassettes. Clonality of isolates was assessed by Pulsed-field gel electrophoresis (PFGE) method. The ctx, zot, and tcp genes were present in 100 % of isolates. The wbeT gene was absent in all V. cholerae outbreak isolates, integrity of which is essential for Ogawa phenotype. This correlates with Inaba phenotype of all isolates under study. Sequencing of the ctxB + strains revealed that all isolates (El Tor strains) possessed the ctxB sequence of classical biotype allele known as El Tor variant strains. No class 1 or 2 integrons were detected among the isolates which indicate that in spite of high rate of resistance, integrons do not play an important role in V. cholerae resistance. All isolates were chloramphenicol sensitive all of which showed resistance to tetracycline and harbored the tetB resistance gene. PFGE analysis showed identical pulsotypes indicative of clonal dissemination of a single V. cholerae strain among the patients under study. Clonal cholera outbreak in boarder cities is alarming due to fear of import and spread of V. cholerae strains from out of the country which may lead to more spreading epidemics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vibrio cholerae is a gram-negative bacterium which causes cholera, a life-threatening illness, especially among children [43]. Different cases of Ogawa and Inaba serogroups of V. cholerae O1 continue to be an important cause of morbidity and mortality in many parts of the Asia and Middle East and have been found during previous years in Iran [8]. Among V. cholerae serotypes, only O1 and O139 have been identified from cholera outbreaks strains. Former studies in Iran have revealed outbreaks and sporadic cases from 1998 to 2011 which caused by V. cholerae O1 Ogawa and Inaba serogroups of El Tor biotype [7, 39, 41].
Epidemic cholera strains (classical or El Tor) cause human illness by acquisition of CTX phage which enables V. cholerae strains to attach and produce cholera toxin within the intestinal lumen. Differentiation of classical and El Tor biotypes of V. cholerae can be done by several phenotypic methods [24]. Moreover, allelic variations in the coding sequence of ctxB, tcpA, and rstR among the biotype strains exist which can be used as molecular markers to characterize CTX phage types and track cholera outbreak strains [51].
There are reports that resistant V. cholerae strains to the commonly used antibiotics are appearing with increasing frequency in India, Iran, and other countries with annually cholera outbreaks [9, 11, 15, 55]. Resistance to antibiotics emerges by mutation or by the acquisition of foreign DNA in the form of plasmids, transposons, or integrons [21, 28].
Nine classes of integrons have been described and based on the integrase gene sequences, two of which (class 1 and 2) being most frequently associated with resistance in clinical infection and play role to the spread of antimicrobial drug resistance in healthcare settings [2, 10, 22].
To characterize V. cholerae strains in Iran, different molecular typing techniques such as ribotyping and PFGE have been used [7, 40]. In this study, we conducted the investigation of differences in ctxB gene among V. cholerae strains obtained from an outbreak in 2013 in Iran. Distribution of tetracycline resistance genes and class 1 and 2 integrons together with PFGE analysis were also investigated to determine the genetic relatedness of isolates.
Materials and Methods
Bacterial Strains
Stool sample of patients suspected to be affected by cholera during summer epidemic which occurred in 2013 in Sistan–Baluchestan province of Iran was submitted to our laboratory, enriched in alkaline peptone water, and incubated at 37 °C for 4 h after which subcultured on TCBS agar medium and incubated at 37 °C for overnight. Colonies suspected to be V. cholerae were subjected to further biochemical tests including sucrose and lactose fermentation, Oxidase test, motility, H2S producing, indole, growth in 0 % NaCl, ornithine decarboxylase, arginine dehydrolase, methyl red, and Voges–Proskauer [12, 50]. The identity of each V. cholerae isolate was confirmed by two species-specific PCR which amplifies within the 16S–23S rRNA intergenic spacer regions and recA genes, respectively, of V. cholerae genome [13, 18].
Serotyping
Serotyping of isolates was performed by polyclonal O1 and O139 and monospecific Ogawa and Inaba antisera with slide agglutination test (Mast Diagnostics Ltd., Bootle, Merseyside, UK).
PCR for Toxin and Resistance Genes
One single colony suspended into 200 µl of sterile distilled water was used for DNA extraction. This suspension boiled for 5 min and after centrifugation at 12,000 rpm 5 µl of supernatant was used as template in each PCR reaction [25].The presence of ctxA and zot (CTX toxin cluster), wbeT (WBE O antigen cluster), tcpA (VPI cluster), tetA, tetB, tetC, tetD (tetracycline resistance genes), and class 1 and 2 integrons was investigated by PCR to characterized the V. cholerae isolates. Each PCR reaction was performed in a 25 µl total reaction volume containing 20 µl sterile water, 2.5 µl 10× Taq polymerase buffer, 0.6 µl MgCl2 (25 mM), 0.3 µl dNTPs (10 mM), 0.5 unit Taq DNA polymerase, and 25 pmol of each primer. V. cholerae O1 classical ATCC 14035 was used as a positive control in each PCR assay. Primer sequences and the PCR cycling conditions are presented in Table 1.
Nucleotide Sequence of Cholera Toxin (ctxB) Gene and GenBank Accession Numbers
The whole ctxB gene was amplified by PCR using specific set of primers which amplify the entire ctxB gene (Table 1). ABI 37309 capillary sequencer (Genfanavaran, Macrogen, Seoul, Korea) was used for direct sequencing of PCR amplified products. The ctxB sequences of V. cholerae O1 El Tor strain N16961 and V. cholerae classical strain ATCC14035 available in the GenBank database were used as references for sequence analysis. One representative of ctxB sequences was deposited in the GenBank database under the accession number KM347971.
Antimicrobial Susceptibility Testing of Isolates
Antimicrobial susceptibility of isolates was performed according to the standard criteria recommended by the world health organization for V. cholerae [36]. Each isolate was tested for susceptibility to 5 antimicrobial agents: tetracycline (30 µg), ciprofloxacin (5 µg), cotrimoxazole (25 µg), nalidixic acid (30 µg), and chloramphenicol (30 µg) (Difco Laboratories, Detroit,). Escherichia coli ATCC 25922 was used as quality control in each antimicrobial susceptibility assay.
Pulsed-Field Gel Electrophoresis Genotyping of V. cholerae Isolates
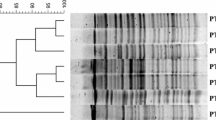
Genotyping of V. cholerae isolates was performed by PFGE method according to PulseNet standardized protocol [14]. Briefly, NotI restriction enzyme (Roche Diagnostic GmbH, Mannheim, Germany) was used for digestion of the genomic DNA of isolates which was electrophoresed in the condition consisting of block 1 with an initial switch time (IST) of 2 s to final switch time (FST) of 10 s and a run time of 13 h, and block 2 with an IST of 20 s to a FST of 25 s with a run time of 6 h and a gradient of 6.0 V/cm and an included angle of 120° at 14 °C. The XbaI-digested Salmonella serotype Brander up H9812 was used as DNA molecular weight size marker for analysis of banding patterns (Fig. 1).
Results
Identification of Strains and Serotyping
Thirty-three isolates of V. cholerae with yellow colonies on TCBS agar medium, oxidase+, and motility+ were included in this study. PCR assay with specific primers for V. cholerae 16S-23S rRNA gene confirmed the identity of isolates. Voges–Proskauer test showed El Tor biotype for all isolates. All 33 strains were agglutinated with O1-Polyvalent and Inaba monospecific antisera and identified as V. cholerae O1, biotype El Tor, and serotype Inaba.
Antimicrobial Resistance Profile
A high rate of resistance (100 %) was seen to tetracycline, Nalidixic acid, and cotrimoxazole. All isolates were sensitive to chloramphenicol and ciprofloxacin.
PCR Assay for Toxin and Resistance Genes
PCR assay showed that all isolates possessed tcpA and ctxB genes. None of isolates harbored wbeT gene and the genes associated with class 1 or class 2 integrons. Of tetracycline resistance genes, tetB was found in all isolates. No tetD, tetC, or tetA genes were detected.
Nucleotide Sequencing of ctxB
Sequencing of the ctxB + strains revealed that all isolates in this study (El Tor strains) possessed the ctxB sequence of classical biotype allele. They have cytosine nucleotide at the position 115 and 203 comparing to classical ATCC 14035 references strain and should be reported as El Tor variant strains [35, 37, 45].
PFGE Analysis
The genetic relationship among 33 V. cholerae isolates was determined by PFGE using NotI (5′-GCGGCCGC-3′) restriction digestion of chromosomal DNA. All 33 isolates showed identical pulsotypes indicative of clonal dissemination of a single V. cholerae strain among the patients under study. Restriction digestion banding pattern of isolates ranged from <1000 to >20 kb with approximately 21 visible bands for each isolate.
Discussion
All isolates in this study were characterized as V. cholerae O1 Inaba with El Tor biotype. Previous studies in Iran have reported the dominance of Inaba serotype strains in Iran during 2005–2010 followed by a nationwide cholera due to Ogawa strains, which was occurred in Iran during 2011. In this study, all El Tor isolates harbored the ctxB sequence of classical biotype which consequently describes them as El Tor variants [35, 38, 45].Our previously published data have defined the appearance and dominance of El Tor variants in Iran in 2004 through 2012 [3, 6].
Likewise, the El Tor variant strains were also reported in numerous countries [29, 30, 32, 34, 45, 46], which are an indication of the ongoing changes in the sequence of ctxB genes in both El Tor and classical strains [4, 27]. This proposes the hypothesis that probably El Tor variants are more compatible with the human and environmental conditions and are developing as a dominant genotype worldwide.
PCR analysis of clinical isolates showed that ctx, zot, and tcp genes were present in 100 % of isolates, which further emphasizes on their crucial role in V. cholerae virulence and pathogenicity.
The product of wbeT gene has a transferase activity, and its integrity is mandatory for Ogawa phenotype. The wbeT gene was absent in all V. cholerae outbreak isolates which may be probably due to (i) deletion of WbeT gene or (ii) mutations within the primer annealing sequences which would be easily misjudged as negative results. All 33 V. cholerae, O1 isolates under study belonged to Inaba serotype which correlates with the absence of an intact wbeT gene in O-antigen biosynthesis cluster.
In this study, no class 1 or 2 integrons were detected among the isolates which is comparable with the former studies in Iran, in which the low contribution of integrons was shown among clinical O1 isolates from 2004 to 2006 and environmental non-O1/non-O139 isolates from surface waters [1, 5]. Reports from India and Thailand also declare the low distribution of these genetic elements among their clinical or environmental V. cholerae isolates [16, 49]. These data indicate that in spite of the high rate of resistance, integrons do not play an important role in V. cholerae resistance. Although, the significant role of integrons in conferring resistance to other enteric gram-negative bacteria should not be ignored.
Genotyping analysis of isolates showed an identical pulsotype which is indicative of a high level of homogeneity. Regarding the Afghan nationality of 85 % of patients, all of which live in refugee camps, it is strongly suggestive of clonal dissemination of a single strain among the patients. Formerly, we have also reported a low diversity among cholera epidemic strains (2011) which was occurred in 16 province of Iran [20]. Moreover, clonal relationship among V. cholerae strains isolated in Iran was repeatedly reported from this country [6, 7].
In this study all isolates were sensitive to chloramphenicol and ciprofloxacin. Comparing the results with previous studies in the country shows that resistance to chloramphenicol has been decreased, which may evidently reflect the improvement in prescription of suitable antibiotics by physicians. However, the clonal nature of isolates may cause a misestimating [1, 42].
High resistance to cotrimoxazole is a common feature among V. cholerae in Iran and in many countries encountering cholera epidemics. Previous reports from Iran, India, and China have also shown a resistance rate of 74, 100, and 83.4 % against cotrimoxazole [17, 47, 56]. In many of previous studies, high resistance to cotrimoxazole was strongly attributed to SXT element which confers simultaneous resistance to cotrimoxazole, streptomycin, and chloramphenicol. In the present study, all of isolates were chloramphenicol sensitive which rules outs the contribution of SXT element and suggests the involvement of other resistance conferring genetic mechanisms [1, 23, 42, 44].
Ninety percent of isolates showed resistance to tetracycline and harbored the tetB resistance gene. This high resistance to tetracycline was also reported by previous studies in this region in 2005 and 2012 but unfortunately, no pervious study has reported the incidence of tetracycline resistance gene in V. cholera isolates in Iran [40, 47]. Kim and colleagues and Furushita et al. found the tetB gene among V. cholera isolated from marine aquatic farms of Korea and heterogeneous gram-negative strains isolated from fish-farm bacteria with the percentage of 100 and 72 %, respectively [19, 26].
In conclusion, among tetracycline resistance genes, tetB is more widely distributed among V. cholerae isolates. The El Tor variant strains show an ongoing increase in Iran and probably are developing as a dominant genotype worldwide. Cholera outbreaks in boarder cities are alarming due to fear of import and spread of V. cholerae strains from out of the country, which may lead to more spreading epidemics.
References
Adabi M, Bakhshi B, Goudarzi H, Zahraei SM, Pourshafie MR (2009) Distribution of class I integron and sulfamethoxazole trimethoprim constin in Vibrio cholerae isolated from patients in Iran. Microb Drug Resist 15(3):179–184. doi:10.1089/mdr.2009.0885
Albert MJ, Rotimi VO, Dhar R, Silpikurian S, Pacsa AS, Molla AM, Szucs G (2009) Diarrhoeagenic Escherichia coli are not a significant cause of diarrhoea in hospitalised children in Kuwait. BMC Microbiol 9:62. doi:10.1186/1471-2180-9-62
Aliabad NH, Bakhshi B, Pourshafie MR, Sharifnia A, Ghorbani M (2012) Molecular diversity of CTX prophage in Vibrio cholerae. Lett Appl Microbiol 55(1):27–32. doi:10.1111/j.1472-765X.2012.03253.x
Ansaruzzaman M, Bhuiyan N, Nair GB, Sack DA, Lucas M, Deen JL, Ampuero J, Chaignat C-L, Group MCVDPC (2004) Cholera in Mozambique, variant of Vibrio cholerae. Emerg Infect Dis 10(11):2057
Bakhshi B, Barzelighi HM, Adabi M, Lari AR, Pourshafie MR (2009) A molecular survey on virulence associated genotypes of non-O1 non-O139 Vibrio cholerae in aquatic environment of Tehran, Iran. Water Res 43(5):1441–1447. doi:10.1016/j.watres.2008.12.025
Bakhshi B, Boustanshenas M, Mahmoudi-Aznaveh A (2014) Emergence of Vibrio cholerae O1 classical biotype in 2012 in Iran. Lett Appl Microbiol 58(2):145–149. doi:10.1111/lam.12167
Bakhshi B, Pourshafie MR (2009) Assessing clonality of Vibrio cholerae strains isolated during four consecutive years (2004–2007) in Iran. Scand J Infect Dis 41(4):256–262. doi:10.1080/00365540902767049
Bakhshi B, Pourshafie MR, Navabakbar F, Tavakoli A (2008) Genomic organisation of the CTX element among toxigenic Vibrio cholerae isolates. Clin Microbiol Infect 14(6):562–568. doi:10.1111/j.1469-0691.2008.01976.x
Basu A, Garg P, Datta S, Chakraborty S, Bhattacharya T, Khan A, Ramamurthy S, Bhattacharya SK, Yamasaki S, Takeda Y, Nair GB (2000) Vibrio cholerae O139 in Calcutta, 1992-1998: incidence, antibiograms, and genotypes. Emerg Infect Dis 6(2):139–147. doi:10.3201/eid0602.000206
Cambray G, Guerout AM, Mazel D (2010) Integrons. Annu Rev Genet 44:141–166. doi:10.1146/annurev-genet-102209-163504
Chakraborty S, Garg P, Ramamurthy T, Thungapathra M, Gautam JK, Kumar C, Maiti S, Yamasaki S, Shimada T, Takeda Y, Ghosh A, Nair GB (2001) Comparison of antibiogram, virulence genes, ribotypes and DNA fingerprints of Vibrio cholerae of matching serogroups isolated from hospitalised diarrhoea cases and from the environment during 1997-1998 in Calcutta, India. J Med Microbiol 50(10):879–888
Choopun N, Louis V, Huq A, Colwell RR (2002) Simple procedure for rapid identification of Vibrio cholerae from the aquatic environment. Appl Environ Microbiol 68(2):995–998
Chun J, Huq A, Colwell RR (1999) Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl Environ Microbiol 65(5):2202–2208
Cooper K, Luey C, Bird M, Terajima J, Nair G, Kam K, Arakawa E, Safa A, Cheung D, Law C (2006) Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog Dis 3(1):51–58
Dalsgaard A, Forslund A, Bodhidatta L, Serichantalergs O, Pitarangsi C, Pang L, Shimada T, Echeverria P (1999) A high proportion of Vibrio cholerae strains isolated from children with diarrhoea in Bangkok, Thailand are multiple antibiotic resistant and belong to heterogenous non-O1, non-O139 O-serotypes. Epidemiol Infect 122(2):217–226
Dalsgaard A, Forslund A, Serichantalergs O, Sandvang D (2000) Distribution and content of class 1 integrons in different Vibrio cholerae O-serotype strains isolated in Thailand. Antimicrob Agents Chemother 44(5):1315–1321
Das S, Saha R, Kaur IR (2008) Trend of antibiotic resistance of Vibrio cholerae strains from East Delhi. Indian J Med Res 127(5):478–482
Dashtbani-Roozbehani A, Bakhshi B, Pourshafie MR (2013) Genetic relatedness of clinical and environmental Vibrio cholerae isolates based on triple housekeeping gene analysis. Curr Microbiol 67(1):15–20. doi:10.1007/s00284-013-0324-7
Furushita M, Shiba T, Maeda T, Yahata M, Kaneoka A, Takahashi Y, Torii K, Hasegawa T, Ohta M (2003) Similarity of tetracycline resistance genes isolated from fish farm bacteria to those from clinical isolates. Appl Environ Microbiol 69(9):5336–5342
Hajia M, Rahbar M, Rahnamye Farzami M, Masoumi Asl H, Dolatyar A, Imani M, Saburian R, Mafi M, Bakhshi B (2015) Assessing Clonal Correlation of Epidemic Vibrio cholerae Isolates During 2011 in 16 Provinces of Iran. Curr Microbiol 70(3):408–414. doi:10.1007/s00284-014-0725-2
Hall RM, Collis CM (1995) Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol 15(4):593–600
Iyer A, Barbour E, Azhar E, Salabi AAE, Hani Mutlak A, Hassan IQ, Adeel Chaudhary AA, Kumosani T, Damanhouri G, Alawi M, Na’was T, Nour AMA, Harakeh S (2013) Transposable elements in Escherichia coli antimicrobial resistance. Adv Biosci Biotechnol 4:415–423
Jain M, Goel AK, Bhattacharya P, Ghatole M, Kamboj DV (2011) Multidrug resistant Vibrio cholerae O1 El Tor carrying classical ctxB allele involved in a cholera outbreak in South Western India. Acta Trop 117(2):152–156. doi:10.1016/j.actatropica.2010.12.002
Kaper JB, Morris JG Jr, Levine MM (1995) Cholera. Clin Microbiol Rev 8(1):48–86
Karaolis DK, Lan R, Reeves PR (1995) The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol 177(11):3191–3198
Kim YH, Jun LJ, Park SH, Yoon SH, Chung JK, Kim JC, Jeong HD (2007) Prevalence of tet(B) and tet(M) genes among tetracycline-resistant Vibrio spp. in the aquatic environments of Korea. Dis Aquat Organ 75(3):209–216. doi:10.3354/dao075209
Kumar P, Jain M, Goel A, Bhadauria S, Sharma S, Kamboj D, Singh L, Ramamurthy T, Nair G (2009) A large cholera outbreak due to a new cholera toxin variant of the Vibrio cholerae O1 El Tor biotype in Orissa. Eastern India. J Med Microbiol 58(2):234–238
Mazel D, Davies J (1999) Antibiotic resistance in microbes. Cell Mol Life Sci 56(9–10):742–754
Mercy N, Mohamed AA, Zipporah N, Chowdhury G, Pazhani GP, Ramamurthy T, Boga HI, Kariuki SM, Joseph O (2014) Phenotypic and genetic characterization of vibrio cholerae O1 isolated from various regions of Kenya between 2007 and 2010. Pan Afr Med J 19:8. doi:10.11604/pamj.2014.19.8.2496
Morita M, Ohnishi M, Arakawa E, Yamamoto S, Nair GB, Matsushita S, Yokoyama K, Kai A, Seto K, Watanabe H, Izumiya H (2010) Emergence and genetic diversity of El Tor Vibrio cholerae O1 that possess classical biotype ctxB among travel-associated cases of cholera in Japan. J Med Microbiol 59(Pt 6):708–712. doi:10.1099/jmm.0.017624-0
Mukhopadhyay AK, Chakraborty S, Takeda Y, Nair GB, Berg DE (2001) Characterization of VPI pathogenicity island and CTXphi prophage in environmental strains of Vibrio cholerae. J Bacteriol 183(16):4737–4746. doi:10.1128/jb.183.16.4737-4746.2001
Nair GB, Qadri F, Holmgren J, Svennerholm AM, Safa A, Bhuiyan NA, Ahmad QS, Faruque SM, Faruque AS, Takeda Y, Sack DA (2006) Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol 44(11):4211–4213. doi:10.1128/JCM.01304-06
Ng LK, Mulvey MR, Martin I, Peters GA, Johnson W (1999) Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella serovar Typhimurium DT104. Antimicrob Agents Chemother 43(12):3018–3021
Nguyen BM, Lee JH, Cuong NT, Choi SY, Hien NT, Anh DD, Lee HR, Ansaruzzaman M, Endtz HP, Chun J, Lopez AL, Czerkinsky C, Clemens JD, Kim DW (2009) Cholera outbreaks caused by an altered Vibrio cholerae O1 El Tor biotype strain producing classical cholera toxin B in Vietnam in 2007 to 2008. J Clin Microbiol 47(5):1568–1571. doi:10.1128/JCM.02040-08
Olsvik O, Wahlberg J, Petterson B, Uhlen M, Popovic T, Wachsmuth IK, Fields PI (1993) Use of automated sequencing of polymerase chain reaction-generated amplicons to identify three types of cholera toxin subunit B in Vibrio cholerae O1 strains. J Clin Microbiol 31(1):22–25
Perilla M, Ajello G, Bopp C, Elliott J, Facklam R (2003) Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. Haemophilus influenzae Neisseria meningitidis Streptococcus pneumoniae Neisseria gonorrhoeae Salmonella serotype Typhi Shigella and Vibrio cholerae
Popovic T, Fields PI, Olsvik O (1994) Detection of cholera toxin genes. Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, pp 41–52
Popovic TFP, Olsvik O (1994) Vibrio cholerae and cholera: molecular to global perspectives. In: Wachsmuth IK, Blake PA, Olsvik O (eds) Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, pp 41–52
Pourshafie M, Grimont F, Kohestani S, Grimont PA (2002) A molecular and phenotypic study of Vibrio cholerae in Iran. J Med Microbiol 51(5):392–398
Pourshafie MR, Bakhshi B, Ranjbar R, Sedaghat M, Sadeghifard N, Zaemi Yazdi J, Parzadeh M, Raesi J (2007) Dissemination of a single Vibrio cholerae clone in cholera outbreaks during 2005 in Iran. J Med Microbiol 56(Pt 12):1615–1619. doi:10.1099/jmm.0.47218-0
Pourshafie MR, Grimont F, Saifi M, Grimont PA (2000) Molecular epidemiological study of Vibrio cholerae isolates from infected patients in Teheran, Iran. J Med Microbiol 49(12):1085–1090
Rahmani F, Fooladi AAI, Marashi SMA, Nourani MR (2012) Drug resistance in Vibrio cholerae strains isolated from clinical specimens. Acta Microbiol Immunol Hung 59(1):77–84
Ramamurthy T, Yamasaki S, Takeda Y, Nair GB (2003) Vibrio cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect 5(4):329–344
Ranjbar M, Rahmani E, Nooriamiri A, Gholami H, Golmohamadi A, Barati H, Rajabifar D, Barati S, Sabet MS, Zamiri A, Haghighi S, Taifehashemi P, Nojomi M (2010) High prevalence of multidrug-resistant strains of Vibrio cholerae, in a cholera outbreak in Tehran-Iran, during June-September 2008. Trop Doct 40(4):214–216. doi:10.1258/td.2010.100015
Raychoudhuri A, Patra T, Ghosh K, Ramamurthy T, Nandy RK, Takeda Y, Balakrish-Nair G, Mukhopadhyay AK (2009) Classical ctxB in Vibrio cholerae O1, Kolkata, India. Emerg Infect Dis 15(1):131–132. doi:10.3201/eid1501.080543
Safa A, Sultana J, Dac Cam P, Mwansa JC, Kong RY (2008) Vibrio cholerae O1 hybrid El Tor strains, Asia and Africa. Emerg Infect Dis 14(6):987–988. doi:10.3201/eid1406.080129
Sedaghat M, Rahimi F, Talebi M, Pourshafie MR (2013) Serotyping, Antibiotic Susceptibility Pattern and Detection of hlyA Gene Among Cholera Patients in Iran. Jundishapur J Microbiol 6(1):20–23
Sharifnia A, Bakhshi B, Pourshafie MR (2012) wbeT sequence typing and IS1004 profiling of Vibrio cholerae isolates. Lett Appl Microbiol 54(4):267–271. doi:10.1111/j.1472-765X.2012.03204.x
Shi L, Fujihara K, Sato T, Ito H, Garg P, Chakrabarty R, Ramamurthy T, Nair GB, Takeda Y, Yamasaki S (2006) Distribution and characterization of integrons in various serogroups of Vibrio cholerae strains isolated from diarrhoeal patients between 1992 and 2000 in Kolkata, India. J Med Microbiol 55(Pt 5):575–583. doi:10.1099/jmm.0.46339-0
Stavric S, Bachanan B (1995) The Isolation and Identification of V. cholerae O1 and non-O1 from Foods. Polyscience Publication, Government of Canada, Health Protection Branch, Ottawa (MFLP-72), Canada
Talkington D, Bopp C, Tarr C, Parsons MB, Dahourou G, Freeman M, Joyce K, Turnsek M, Garrett N, Humphrys M (2011) Characterization of toxigenic Vibrio cholerae from Haiti, 2010–2011. Emerg Infect Dis 17(11):2122
Tamayo M, Koblavi S, Grimont F, Castaneda E, Grimont P (1997) Molecular epidemiology of Vibrio cholerae O1 isolates from Colombia. J Med Microbiol 46(7):611–616
Waters SH, Rogowsky P, Grinsted J, Altenbuchner J, Schmitt R (1983) The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res 11(17):6089–6105
White PA, McIver CJ, Rawlinson WD (2001) Integrons and gene cassettes in the enterobacteriaceae. Antimicrob Agents Chemother 45(9):2658–2661
Yamamoto T, Nair GB, Takeda Y (1995) Emergence of tetracycline resistance due to a multiple drug resistance plasmid in Vibrio cholerae O139. FEMS Immunol Med Microbiol 11(2):131–136
Yu L, Zhou Y, Wang R, Lou J, Zhang L, Li J, Bi Z, Kan B (2012) Multiple antibiotic resistance of Vibrio cholerae serogroup O139 in China from 1993 to 2009. PLoS One 7(6):e38633
Acknowledgments
This study was supported by a Grant from Research council of Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakhshi, B., Mahmoudi-Aznaveh, A. & Salimi-Khorashad, A. Clonal Dissemination of a Single Vibrio cholerae O1 Biotype El Tor Strain in Sistan–Baluchestan Province of Iran During 2013. Curr Microbiol 71, 163–169 (2015). https://doi.org/10.1007/s00284-015-0806-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0806-x