Abstract
Endophytic fungi are ubiquitous in the plant kingdom and they produce a variety of secondary metabolites to protect plant communities and to show some potential for human use. However, secondary metabolites produced by endophytic fungi in the medicinal plant Curcuma wenyujin are sparsely explored and characterized. The aim of this study was to characterize the secondary metabolites of an active endophytic fungus. M7226, the mutant counterpart of endophytic fungus EZG0807 previously isolated from the root of C. wenyujin, was as a target strain. After fermentation, the secondary metabolites were purified using a series of purification methods including thin layer chromatography, column chromatography with silica, ODS-C18, Sephadex LH-20, and macroporous resin, and were analyzed using multiple pieces of data (UV, IR, MS, and NMR). Five compounds were isolated and identified as curcumin, cinnamic acid, 1,4-dihydroxyanthraquinone, gibberellic acid, and kaempferol. Interestingly, curcumin, one of the main active ingredients of C. wenyujin, was isolated as a secondary metabolite from a fungal endophyte for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Curcuma wenyujin Y.H.Chen and C.Ling is a well-known species used in traditional Chinese medicine [10]. It synthesizes an array of volatile compounds including curcumin, curcumol, β-elemene, curzerene, and curzerenone that have special pharmacological effects on bacterial infections, cervical cancer, and acesodyne, which make C. wenyujin a highly valuable Chinese traditional medicinal plant [3, 25]. In 2005, the oil of this plant was regarded as antitumor and antibacterial drug by the State Pharmacopoeia Commission [11].
Endophytic fungi are microorganisms that colonize the tissues of healthy plants without causing apparent harm. They are important in plant growth and development in keeping their ability of secondary metabolite production and their ability to colonize all types of host tissues and organs [12]. In addition, medicinal plants are recognized as a repository of fungal endophytes with active metabolites of pharmaceutical importance [17]. Earlier studies reported that endophytic fungi in Chinese traditional plants are potential sources for the production of antimicrobial, antitumoural ,and other bioactive secondary metabolites [27]. Interestingly, it has been reported that fungal endophytes might synthesize metabolites similar to or even more active than those produced by their hosts [15]. Therefore, studying secondary metabolites produced by C. wenyujin endophytes will not only aid in the search for useful compounds, but also play an important role in protection of medicinal plant resources.
We previously screened a bioactive strain M7226, the mutant counterpart of endophytic fungus EZG0807 isolated from the root of C. wenyujin, characterized its rDNA sequence of this mutant using molecular techniques and deposited this sequence to the GenBank with the accession number JQ003920 [22]. This characterized endophytic fungal mutant was later identified as Gibberella fujikuroi (Sawada) Wollenw (Fusarium moniliforme) and was found to synthesize secondary metabolites producing a broad spectrum of antibacterial activities. The secondary metabolites produced by fungal endophytes in C. wenyujin are sparsely explored and this study was aimed to characterize the secondary metabolites produced by M7226.
Materials and Methods
Fermentation, Extraction, and Compounds Isolation
M7226 was grown on PDA at 28 °C for 3 days. Then three pieces (5 × 5 mm2) of mycelial agar plugs were inoculated in Czapek medium (3.0 g/l NaNO3, 1.0 g/l K2HPO4, 0.5 g/l MgSO4·7H2O, 0.5 g/l KCl, 0.01 g/l FeSO4·7H2O, 30 g/l sucrose) at 28 °C in a shaker at 160 rpm for 10 days. The harvested broth culture was centrifuged at 12, 000 rpm for 15 min to separate supernatant and mycelia. The mycelia were extracted with acetone for 3 days and the extract was combined with the supernatant. The final solution was successively extracted with petroleum ether, chloroform, ethyl acetate, and n-butanol. Extracts with antibacterial activity were further purified by a series of separation chromatography methods including thin layer chromatography, column chromatography with silica, ODS-C18, Sephadex LH-20, and macroporous resin (Fig. 1).
Antibacterial Assay
Antimicrobial assays were performed in vitro using the disk diffusion method following the procedure of Gaudreau and Gilbert [4] against Escherichia coli Migula (ATCC25922), Proteus vulgaris Hauser (ATCC3851), Bacillus subtilis Ehrenberg (ATCC6633), and Staphylococcus aureus Rosenbach (ATCC25923). The assay was performed in triplicate, and results were expressed as mean ± SD. Statistic analysis was carried out by one-factor variance analysis with the Data Processing System software (DPS 7.05).
Structure Elucidation
Ultraviolet (UV) absorption spectra were measured on an Ultrospec 4300 pro spectrophotometer (Amersham Biosciences). Infrared spectra (IR) were recorded on an IR Prestige-21 spectrometer (Shimadzu Japan). Mass spectra were carried out using a mass spectrometer ESI–MS (Thermo–Finnigan). Nuclear magnetic resonance (NMR) spectra were acquired in Bruker Avance spectrometers (Bruker), working at 500 MHz for 1H and 125 MHz for 13C.
Results
After fermentation of M7226 for 10 days, a total of 45 l fungi-free culture supernatants were harvested and extracted with petroleum ether, chloroform, ethyl acetate, and n-butanol, respectively. The extract obtained with the culture medium (blank) was assessed in antibacterial assays and did not present any significant activity. The chloroform and n-butanol extracts showed overt antibacterial activity; while extracts obtained with petroleum ether and ethyl acetate were inactive. Therefore, the crude chloroform extract (5.213 g) was separated by silica column chromatography (100–200 mesh), eluting with a chloroform–methanol gradient (1:0, 99:1, 95:5, 9:1, 4:1, 0:1) to yield five fractions (A, B, C, D, E). Fractions A, C, and E were of no interest according to TLC data. Fraction B (327 mg) was further purified on silica gel using chloroform–methanol mixtures of increasing polarity (200:1, 100:1, 50:1, 20:1, 10:1), followed by gel filtration on a Sephadex LH-20 to yield compounds 1 (12.6 mg) and 2 (15.1 mg). Repeated chromatography on fraction D on silica gel (200–300 mesh; CHCl3/MeOH 100:1→10:1) followed by pre-coated TLC using CHCl3/MeOH (1:1) were used to isolate compound 3 (17.0 mg).
Concentration of the n-butanol extract under reduced pressure resulted in a brown residue (17.0 g), which was dissolved in H2O and insoluble substances were discarded. The hydro-soluble fraction (15.8 g) was subjected to column chromatography over the macroporous resin D101, eluting with a gradient of H2O 30, 50, 70, and 95 % ethanol. After the eluate obtained with 30 % ethanol was concentrated, the sample was submitted to gel filtration on Sephadex LH-20 with MeOH–H2O as eluent, followed by separation on ODS-C18 column (12 nm S-50 μm; MeOH/H2O) to yield compound 4 (23.4 mg). Repeated chromatography of the eluate obtained with 50 % ethanol on ODS-C18 column (MeOH/H2O 100:1→10:1) followed by gel filtration on Sephadex LH-20 with MeOH/H2O (1:1) as mobile phase resulted in compound 5 (11.3 mg). The schematic diagram of compound isolation is shown in Fig. 1.
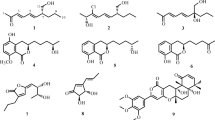
The structures of these compounds were elucidated by UV, IR, MS, 1H, and 13C NMR spectrometry and are shown in Fig. 2. Compound 1 was isolated as orange powder, which was soluble in methanol, ethanol, and chloroform. It showed a maximum UV absorption bands at 421 nm in ethanol. The stretching vibrations at 3,300, 1,641, and 1,519 cm−1, characteristic absorption peaks of curcumin, were observed in compound 1’s IR spectrum [18]. The NMR spectroscopic data were: 1H NMR (500 MHz, DMSO-d 6) δ 3.83 (6H, s, –OCH3), 6.08 (2H, s, –CH2–), 6.77 (2H, d, J = 15.8 Hz, CO–CH=), 6.82 (2H, dd, J = 7.5, 6.5 Hz, Ar–H 5′), 7.16 (2H, d, J = 8.3 Hz, Ar–H 6′), 7.31 (2H, d, J = 1.7 Hz, Ar–H 2′), 7.56 (2H, m, Ar–CH=); 13C NMR (125 MHz, DMSO-d 6) δ 55.16, 100.39, 110.77, 115.40, 120.55, 122.59, 125.81, 139.84, 147.47, 148.80, and 182.67 ppm. These properties were compared with the Spectral Database for Organic Compounds (SDBS) available at the website (http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgilang=eng) and compound 1 was identified as curcumin.
Compound 2 was a white solid, soluble in methanol, ethanol, and chloroform. It exhibited two UV absorption peaks at 222 and 261 nm in ethanol. The O–H, C=O, C=C, C–H bond, and the benzene skeletal vibration were observed at 3,300–2,500, 1,700–1,660, 1,680–1620, 770–680, and 1,600–1,450 cm−1, respectively, in its IR spectrum. Its ESI–MS showed a strong peak at m/z 147 [M−H], indicating a molecular formula of C9H8O2 and a molecular weight of 148, taking into account the above characteristics. The NMR spectroscopic data were: 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 16 Hz, 1H, H-3), 7.59 (d, J = 3 Hz, 2H, H-2′, 6′), 7.44 (m, 3H, H-3′, 4′, 5′), 6.50 (d, J = 16 Hz, 1H, H-2); 13C NMR (125 MHz, CDCl3) δ 171.40 (C–1), 146.12 (C–3), 133.08 (C–1′), 129.76 (C–4′), 127.98 (C–3′, 5′), 127.39 (C–2′, 6′) and 116.33 (C–2). These properties suggested compound 2 to be cinnamic acid, according to previous data [20] and the SDBS Database.
Compound 3, isolated as orange solid, was soluble in methanol, ethanol ,and chloroform. It exhibited UV absorption peaks in ethanol at 206.4, 225.1, 248.6, 278.7 ,and 473.6 nm. The aromatic ring absorption was found at 1,587 cm−1 in the IR spectrum. The NMR spectroscopic data were: 1H NMR (500 MHz, CDCl3) δ 12.89 (s, 1H, –OH), 8.35 (d, J = 6 Hz, 1H, H-5, 8), 7.85 (d, J = 6 Hz, 1H, H-6, 7), 7.31 (s, 1H, H-2, 3); 13C NMR (125 MHz, CDCl3) δ 185.87 (C–9, 10), 156.80 (C–1, 4), 133.48 (C–6, 7), 132.44 (C–11, 12), 128.35 (C–2, 3), 126.03 (C–5, 8), 111.74 (C–13, 14). The above characteristics indicated that compound 3 had a symmetrical structure. These data were in accordance with the properties of 1,4-dihydroxyanthraquinone in the SDBS spectral database.
Compound 4 displayed a molecular weight of 346 as revealed by the ESI–MS m/z 345 [M−H]. It was isolated as white solid and dissolved easily in methanol and ethanol, but hardly in chloroform. Compound 4 exhibited UV absorption peaks in ethanol at 217 and 253 nm. The NMR spectroscopic data were: 1H NMR (500 MHz, DMSO-d 6) δ 6.33 (1H, d, J = 9.5 Hz, –CH=), 5.80 (1H, dd, J = 9.5 Hz, –CH=), 5.60 (1H, s, –OH), 5.12 (1H, s, =CH2), 4.86 (1H, s, –OH), 4.85 (1H, s, =CH2), 3.87 (1H, s), 3.08 (1H, d, J = 11 Hz, –CH–CO), 2.50 (1H, d, J = 11 Hz), 2.20 (2H, m, –CH2–), 2.11 (1H, m, –CH2–), 1.92 (1H, m, –CH2–), 1.87 (2H, m, –CH2–), 1.73 (2H, m, –CH2–), 1.63 (1H, m, –CH2–), 1.07 (3H, s, –CH3); 13C NMR (125 MHz, DMSO-d 6) δ 178.22 (C-1), 172.61 (C-2), 157.07 (C-3), 132.73 (C-4), 130.95 (C-5), 105.74 (C-6), 90.05 (C-7), 76.09 (C-8), 67.91 (C-9), 52.56 (C-10), 51.59 (C-11), 50.31 (C-12), 49.90 (C-13), 48.88 (C-14), 43.76 (C-15), 42.19 (C-16), 16.01 (C-17), 13.96 (C-18). These properties were in good accordance with gibberellic acid properties found in the SDBS spectral database.
Compound 5, a yellow powder, was easily dissolved in methanol and ethanol, but hardly in chloroform and water. It showed UV absorption peaks in ethanol at 210, 267, and 363 nm. The hydroxyl and carboxyl absorption bands on IR spectrum were found at 3,139 and 1,656 cm−1, respectively, while a benzene ring absorption band was observed at 1,505 and 1,439 cm−1. The deduced chemical formula for compound 5 was C15H10O6, taking into account the ESI–MS peak at m/z 285 [M−H]. The NMR spectroscopic data were: 1H NMR (500 MHz, DMSO-d 6) δ 12.47 (s, 1H, –OH), 8.04 (d, J = 9.0 Hz, 2H, H-2′, 6′), 6.93 (d, J = 9.0 Hz, 2H, H-3′, 5′), 6.44 (d, J = 2.0 Hz, 1H, H-8), 6.19 (d, J = 2.0 Hz, 1H, H-6); 13C NMR (125 MHz, DMSO-d 6) δ 146.30 (C-2), 135.10 (C-3), 175.35 (C-4), 155.66 (C-5), 97.68 (C-6), 163.35 (C-7), 92.97 (C-8), 160.16 (C-9), 102.51 (C-10), 121.14 (C-1′), 128.97 (C-2′, 6′), 114.91 (C-3′, 5′), 158.63 (C-4′). Comparing these characteristics with reference data [26], compound 5 was identified as kaempferol.
Discussion
Curcumin (1), one of the active ingredients of C. wenyujin, was purified from M7226 fermentation, similar to a report by Xuan et al. [21] who isolated two endophytic fungi producing β-elemene, another bioactive component of C. wenyujin, from Curcuma zedoaria. Interestingly, it was isolated as secondary metabolite from an endophyte for the first time. This finding indicated that endophytic fungi can produce the same products as their host, which would not only reduce the need to harvest slow-growing and rare plants but also preserve the world’s ever-diminishing biodiversity.
Cinnamic acid (2), previously isolated from endophytic fungi of long-grain rice (Oryza sativa) [1] and the medicinal plant Melia azedarach [23], has demonstrated antioxidant and antibacterial activities, with a particularly strong inhibition activity on E. coli [19].
Anthraquinones are common secondary metabolites in endophytes fermentation broths [5] and display diverse biological effects, including antibacterial and antitumor activities [2, 9]. Herein, 1,4-dihydroxyanthraquinone (3) was purified. This compound was used as a lead molecule for the design of novel anticancer drugs [8] and exhibited strong growth-inhibition against Clostridium perfringens ATCC13124 [16].
The presence of gibberellic acid (4), important auxin regulating plant physiological processes, in C. wenyujin, could be linked with regulation of plant growth, provision of host plant benefits and synthesis of bioactive metabolites similar as that of host plants [14, 15].
The flavone kaempferol (5), another known metabolite previously isolated from endophytic fungi of long-grain rice (O. sativa) [1], is a markedly active inhibitor of transcriptional activation of COX-2 and showed tyrosinase inhibitory activities, as well as antifungal, anticancer ,and antioxidant activities [7, 24]. Further research is needed to identify the mechanisms by which endophytic fungi synthesize these bioactive molecules [6].
In conclusion, characterization of secondary metabolites of endophytic fungal mutant, M7226 revealed five compounds viz. curcumin, cinnamic acid, 1,4-dihydroxyanthraquinone, gibberellic acid, and kaempferol as important secondary metabolites. These findings suggest that fungal endophytes in C. wenyujin may be potential sources of natural products for exploitation in medicine, agriculture, and industry. Furthermore, it is recognized that microbial sources of valued products may be easier and more economical to produce, effectively reducing their market price. Therefore, we should pay more attention to exploration and utilization of microbial resources, or investment into the microbial Biological Resource Centers [13].
References
Cheng MJ, Wu MD, Hsieh SY et al (2011) Secondary metabolites from the endophytic fungus Annulohypoxylon boveri var. microspora BCRC 34012. Chem Nat Compd 47:536–540
Dhananjeyan MR, Milev YP, Kron MA et al (2005) Synthesis and activity of substituted anthraquinones against a human filarial parasite, Brugia malayi. J Med Chem 48:2822–2830
Ding YL, Xu AX (2005) The research on antitumor activity of Curcuma and its effective constitutes. J Chin Med Mater 28:152–156
Gaudreau C, Gilbert H (1997) Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J Antimicrob Chemother 39:707–712
Ge HM, Tan RX (2009) Symbionts, an important source of new bioactive natural products. Pro Chem 21:30–46
Heinig U, Scholz S, Jennewein S (2013) Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers 60:161–170
Liang YC, Huang YT, Tsai SH et al (1999) Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 20:1945–1952
Lu JR, Kang L, Xin CW et al (2007) Synthesis, characterization and properties of chloro-1,4-dihydroxyanthraquinones. J Tianjin Univ 40:1337–1341
Meazza G, Dayan FE, Wedge DE (2003) Activity of quinones on collectotrichum species. J Agr Food Chem 51:3824–3828
National Pharmacopoeia Committee (2005) Herbs and pieces. The Chinese Pharmacopoeia, 1st edn. Chemical Industry Press, Beijing, pp 194–195
National Pharmacopoeia Committee (2005) Plant oils and extracts. The Chinese Pharmacopoeia, 1st edn. Chemical Industry Press, Beijing, p 279
Scannerini S, Fusconi A, Mucciarelli M (2004) The effect of endophytic fungi on host plant morphogenesis. In: Seckback J (ed) Symbiosis: mechanisms and model systems. Kluwer Academic Publishers, New York, pp 427–447
Smith D, McCluskey K, Stackebrandt E (2014) Investment into the future of microbial resources: culture collection funding models and BRC business plans for biological resource centres. Springerplus 3:81
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216
Strobel GA (2002) Rainforest endophytes and bioactive products. Crit Rev Biotechnol 22:315–333
Sung BK, Kim MK, Lee WH et al (2004) Growth responses of Cassia obtusifolia toward human intestinal bacteria. Fitoterapia 75:505–509
Tejesvi MV, Kini KR, Prakash HS et al (2007) Genetic diversity and antifungal activity of species of Pestalotiopsis isolated as endophytes from medicinal plants. Fungal Divers 24:37–54
Wei LH (2011) Studies on quality and infrared spectrum characteristics of Curcama kwangsinesis. Dissertation, Guangxi University, Guangxi, China
Wells JE, Berry ED, Varel VH (2005) Effects of common forage phenolic acids on Escherichia coli O157:H7 viability in bovine feces. Appl Environ Microbiol 71:7974–7979
Xiao YP, Chen JJ, Zhang YH et al (2004) Studies on the chemical constituents of Fusarium sp. from seagrass endophytic fungus. Chin J Mar Drugs 23:11–13
Xuan Q, Zhang QL, Yang J et al (2011) β-Elemene from Curcuma zedoaria endophytic fungus. Nat Prod Res Dev 23:473–475
Yan JF, Qi NB, Wang SP et al (2013) Identification and biological characteristics of an active endophytic fungus EZG0807. China Biotechnol 33:51–58
Yang SX (2011) Studies on secondary metabolites and their bioactivities of endophytic fungi from Melia Azedarach and Actinomycete from animals. Dissertation, Northwest University of Science and Technology, Shanxi, China
Yang X, Summerhurst DK, Koval SF et al (2001) Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fraction. Phytother Res 15:676–680
Yohei S, Hirozo G, Chihiro T et al (2003) Effects of curcuma drugs on vasomotion in isolated rat aorta. Biol Pharm Bull 26:1135–1143
Yu DQ, Yang JS (1999) Analytical chemistry handbook, vol VII. Chemistey industry Press, Beijing
Zhao JH, Zhang YL, Wang LW et al (2012) Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J Microbiol Biotechnol 28:2107–2112
Acknowledgments
The authors sincerely thank Jianfeng Zhao for providing valuable suggestion for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, J., Qi, N., Wang, S. et al. Characterization of Secondary Metabolites of an Endophytic Fungus from Curcuma wenyujin . Curr Microbiol 69, 740–744 (2014). https://doi.org/10.1007/s00284-014-0647-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0647-z