Abstract
In this study, a naturally unsecretory intrinsically disordered domain of nucleoskeletal-like protein (Nsp) was attempted to be secreted with different types of secretion signals in Bacillus subtilis. The results showed that Nsp can be secreted efficiently by all selected Sec-type signal peptides. Nsp was successfully exported when fused to Tat-type signal peptides but less efficient than Sec-type. The fusion protein with the non-classical extracellular proteins can be detected in the cell and extracellular milieu. This study further demonstrated that the mature protein plays an important role in protein secretion. Moreover, these results indicated that Nsp could be a useful tool to understand the individual roles of mature proteins and signal peptide in protein secretion, to evaluate the effect of conformation of mature proteins on their export pathway when coupled with Tat-type signal peptide, and to seek the signal of non-classical secretory proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of heterologous proteins in secreted form is less than the generation of cytoplasmically localized heterologous proteins in bacteria, but some recombinant proteins expressed in the cytoplasm are frequently limited by their tendency to form inclusion bodies and proteolytic degradation [2]. Protein misfolding and proteolytic degradation can be conveniently countered by means of protein secretion into the culture medium to evade relatively harsh environment of the cytoplasm.
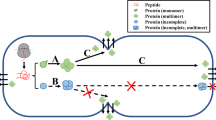
As Bacillus subtilis has a high capacity for secreting its own proteins into the growth medium and is considered as a GRAS organism (Generally Recognized As Safe), it has been investigated intensively with respect to its potential as major “cell factories” for the secretion of heterologous proteins. Newly synthesized preproteins can be targeted to different export pathways in B. subtilis, but most of the preproteins are routed via the Sec or Tat export-dependent pathway. The Sec-dependent preproteins are needed to be in a secretion-competent state during the translocation. By contrast, Tat pathway substrates can be translocated in a folded state. Recently, some cytoplasmic proteins are found to be released in large amounts. This phenomenon termed as non-classical secretion, was first discovered in eukaryotes [7, 15]. Yang et al. have proved that the release of the nonclassical proteins in B. subtilis is a general phenomenon and is not a consequence of cell lysis, but little is known about the mechanism and required conformation for the export of those proteins [16, 17, 21]. The general idea that a previously non-secreted protein can be secreted when a signal peptide fused to amino terminus of this protein is not completely right. For example, LacZ cannot be transported across the membrane by different signal peptides (SPs) [8, 11], while chloramphenicol acetyltransferase is also incompatible for export from B. subtilis [5]. This indicates that mature protein itself may play an important role in protein secretion. However, it is not clear what properties of the substrates are favored by the export pathways and the prediction of suitable SPs for the desired secretion target proteins is impossible.
In reality, not all proteins are structured throughout their entire lengths. Some proteins do not possess definite ordered 3D structure. Such proteins are known as intrinsically disordered proteins (ID) and are characterized by the lack of a fixed 3D structure under physiological conditions. The ID proteins under physiological conditions might contain collapsed and extended disorder [18]. The nuclear pore complexes (NPC) of yeast are composed of about 30 proteins called nucleoporins. These nucleoporins usually contain large intrinsically disordered domains with multiple phenylalanine-glycine repeats (FG domains). Nucleoskeletal-like protein (Nsp1) is one of the nucleoporins featured collapsed and extended disorder in a bimodal distribution along their polypeptide chain [20].
In this study, we explore the possibility of the secretion of intrinsically disordered portion of Nsp1 (Nsp) in B. subtilis using different secretion signals (Sec-type signal peptide, Tat-type signal peptide, and nonclassical secreted proteins), thus to further increase our understanding of the mechanism of protein secretion, and the potential use of Nsp as a tool for the protein translocation studies is discussed.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions
The plasmids and bacterial strains used in this study are listed in Table 1. Escherichia coli DH5α was used as a host for cloning and plasmid manipulations. B. subtilis WB800 served as the protein expression host. They were grown in Luria–Bertani (LB) medium at 37° C.
Plasmids Constructions
The primers used in this study are listed in Online Resource 1 in the supplementary data. The intermediate plasmid pMA5△MCS was constructed as previously described [4]. The encoding sequence of NprE signal peptide was amplified from the genomic DNA of B. subtilis with the corresponding primers. The bgaB gene was amplified using Geobacillus stearothermophilus IAM11001 genomic DNA as the template. The corresponding PCR fragments were digested with NdeI/EcoRI and EcoRI/SacI, respectively, and ligated into NdeI/SacI digested pMA5△MCS, resulting in p-ssNprE-BgaB-his. To construct p-Nsp-his, the fragment of nsp1 gene (from 88 to 1845 bp) was amplified using genomic Baker’s yeast DNA as template and named Nsp. The resulting PCR fragment was digested with NdeI/BamHI and ligated into p-ssNprE-BgaB-his digested with NdeI/BamHI. To generate p-ssNprE-Nsp-his, the fragment of nsp1 gene with EcoRI and BamHI restriction site was amplified by PCR using the corresponding primers. The resulting PCR product was restricted with EcoRI and BamHI, and ligated into EcoRI/BamHI digested p-ssNprE-BgaB-his. To construct the corresponding plasmids, the other selected 29 Sec-dependent signal peptides and two Tat-signal peptides coding sequences were amplified by standard PCR. The PCR products were digested with NdeI/EcoRI, and ligated into NdeI/EcoRI digested p-ssNprE-Nsp-his. As the same way, the plasmids p-GapA-Nsp-his, p-Hag275-Nsp-his, and p-SodA-Nsp-his were constructed.
Transformation of DNA
Competent cells of E. coli DH5α were prepared and transformed as described [6]. Competent cells of B. subtilis WB800 were prepared and transformed with plasmid DNA as described [1].
SDS-PAGE Analysis of Proteins and Western Blotting
Transformants were inoculated into 5 ml Luria–Bertani medium plus kanamycin (50 μg/ml) and grown at 37 °C with shaking for 20 h. The cellular fraction sample and supernatant fraction sample were treated as described previously [21]. Equal volumes of secreted protein fraction and whole cell were applied to the gel and separated by SDS-PAGE. For immunoblot analysis, protein samples were diluted ten times, and after electrophoresis, proteins on the gel were transferred to polyvinylidene difluoride (PVDF) membranes, then was analyzed by the His tag antibody and the western blotting detection kit (TIANGEN) according to the manufacturer’s protocols.
Densitometer Measurements
The quantity of secreted heterologous protein was determined (as a percentage of total secreted proteins from B. subtilis) from analysis of Coomassie Blue stained SDS polyacrylamide gels using a BioRad Model Universal Hood and Quality One computational software (Bio-Rad, CA).
Results
The Secretion of the Unstructured Nsp with Different Sec Signal Peptides
In B. subtilis, the heterologous proteins are commonly secreted via the Sec pathway. Sec-dependent proteins are translocated in an unfolded state. Unfortunately, the vast majority of heterologous proteins are often secreted inefficiently. Various potential bottlenecks in the secretion of heterologous proteins have been proposed. Many of the encountered problems related to the particular properties of the secreted protein, the secretion pathway, or both [12]. In this experiment, we attempted to secrete Nsp with different Sec signal peptides. We used Nsp as a model, because it adopts intrinsically disordered conformations and is naturally unsecreted [20]. Thirty Sec signal peptides were selected to study the secretion of this intrinsically disordered protein Nsp. The corresponding plasmids were constructed to secrete Nsp with different signal peptides. BgaB that cannot be secreted by different Sec signal peptides [19] and Nsp that did not attach to the signal peptides were set as the negative control. After 20-h growth of the transformant in LB medium, the culture supernatant was analyzed by SDS-PAGE (Fig. 1). The obtained quantitative data are shown in Table 2 by densitometer scanning of Coomassie stained gels. As shown in the Fig. 1, Nsp can be hypersecreted by selected signal peptides, although the yield is varied with different signal peptides. BgaB cannot be detected in the supernatants (data not shown). This indicates that Nsp was successfully secreted via the Sec-apparatus but not by cell lysis. Noticeably, the intracellularly expressed Nsp by p-Nsp-his could not be detected in the cytoplasm of B. subtilis or in the supernatant (Fig. 2), this indicated the rapid intracellular degradation of the unstructured Nsp. Additionally, when Nsp was expressed with the Sec-type signal peptides, in all cases no precursor protein was observed in the cytoplasmic fraction (only the signal peptide of NprE was shown in Fig. 2).
The secretion of Nsp by the different signal peptides. The name of signal peptide used for export of Nsp is listed above each lane. The cell fraction (C) of WB800 (p-ssPhoD-Nsp-his), WB800 (p-ssYwbN-Nsp-his), and WB800 (p- Nsp-his) are marked with the letter “C” above the names of signal peptide. In “marker” line, numbers indicate the migration of molecular mass standards (in kilodaltons). Position of precursor Nsp and Nsp are indicated with an arrow
Cellular (C) and supernatant (S) fractions of B. subtilis expressing Nsp at the different signal peptides were immunoblotted. Nsp represent intracellular expression of Nsp using vector p-Nsp-his. Western blots are shown of cellular (C) and supernatant (S) fractions from cells expressing various SP-Nsp fusions (ssYwbN, ssPhoD, ssNprE)
Localization of Nsp Fusions to the Tat-signal Peptides
Opposite to the Sec system that transports unstructured proteins, the recently discovered twin-arginine translocation (Tat) pathway can translocate its protein substrates in a folded state. Due to this feature, the Tat pathway may offer an attractive alternate route for the secretion of heterologous proteins. In B. subtilis, there are two Tat-translocases named TatAdCd and TatAyCy. They exclusively as well as individually export the phosphodiesterase PhoD and the putative heme-containing peroxidase YwbN, respectively [9].
To assess the possibility of using the Tat-type signal peptide to secrete Nsp, the plasmids p-ssPhoD-Nsp-his and p-ssYwbN-Nsp-his were constructed and transformed into B. subtilis WB800. After 20 h of growth, the culture was fractionated into cell and culture supernatant. As shown in Figs. 1 and 2, Nsp protein can be detected in the supernatant, but less than that secreted by Sec-type signal peptide. As same as Sec signal peptide, the precursor protein was not detected in the cellular fraction when YwbN signal peptide was used (Fig. 2). In comparison with Sec and YwbN signal peptide, the unprocessed ssPhoD-Nsp precursor-sized Nsp was found in the cell fraction.
Localization of the Chimeric Nsp Fused to the Non-Classically Secreted Proteins
Recent reviews on protein secretion in B. subtilis lists 24 proteins found in the extracellular environment without typical export signals [17], suggesting that signal peptide independent protein secretion in bacteria is perhaps more common than previously thought. To study whether Nsp can be secreted when fused to the non-classical secreted proteins, three non-classical secreted proteins were used for secretion test. As shown in the Fig. 3, in all three cases, the corresponding bands were detected in the cells and culture supernatant fraction, and the size of the hybrid protein is same in the both location. These results confirmed that fused proteins are transported to the exterior of the cell and the export signal is not removed after translocation.
Discussion
In this study, we have used B. subtilis to secrete intrinsically disordered protein Nsp by different type of secretion signal peptides. It has been found that all the secretion signals mediated the translocation of Nsp into the growth medium. However, significant differences were observed between different signal peptides with respect to yield and Nsp location.
In B. subtilis, vast majority of heterologous proteins export are mediated by Sec-type signal peptides. There are many obstructions in the production of heterologous secretory proteins [12]. The most commonly used strategy to optimize heterologous protein secretion is to find the efficient signal peptide for the desired secretion target protein. Since the best signal peptide for the secretion of one target protein is not automatically the best, or even a sufficient signal peptide, for the secretion of another target protein, many approaches have been established to identify the efficient signal peptides for different target proteins, but up to now the prediction of “good” SPs for the heterologous protein secretion is impossible [3]. We used B. subtilis to secrete the Nsp protein with selected 30 typical Sec-dependent signal peptides. These results showed that all selected Sec-type signal peptides result in high yield Nsp secretion, although the yield at different signal peptides is variable. Previous reports showed that even with different Sec signal peptides, some proteins such as LacZ and BgaB fail to be secreted. These results further demonstrated that the mature protein structure plays an important role in the protein secretion, and the Nsp with intrinsically disordered conformations is favored by the Sec pathway. Since all selected signal peptides can successfully target the disordered Nsp into the Sec machinery, the efficient signal peptides for the secretion of one target protein may be decided by the other role of signal peptide such as retardation of precursor proteins folding. In other words, mature proteins may determine the best signal peptide for its efficient secretion. For the suitable mature proteins, any signal peptides may successfully lead them to the extracellular. The remarkable sequence diversity of signal peptides may result from maintaining the suitable state of mature proteins during secretion. Therefore, the roles of mature proteins translocation may be more critical than currently assumed. Since Nsp is intrinsically disordered and can be secreted at high level with different signal peptide, using this system, it is possible to explore the individual roles of signal peptide and mature proteins for protein secretion in vivo.
In comparison to Sec system, the Tat system is dedicated to the translocation of folded proteins across the bacterial cytoplasmic membrane. Due to its striking characteristic, the Tat pathway might be an attractive alternative for the secretion of heterologous protein [14]. In order to understand whether unstructured Nsp can be secreted when coupled to Tat-signal peptides, the Nsp were fused to the signal peptides of Tat substrates, YwbN and PhoD in B. subtilis. Significant results were obtained compared to the Sec signal peptides secretion. Nsp was inefficiently secreted into the culture supernatant and adverse to the situation found with Sec signal peptide. The preprotien Nsp with the signal peptide of PhoD was observed in the cell fraction. The employment of PhoD and YwbN signal peptides are still insufficient to determine the substrate of the strictly Tat-dependent secretion pathway, because the signal peptides of PhoD and YwbN can direct either Tat- or Sec-dependent secretion of different cargo proteins [10]. However, this cannot rule out the possibility of Nsp secretion by Tat pathway, because the preprotein of Nsp with the Tat-signal peptide was detected intracellularly but not with the Sec signal peptides. In contrast to E. coli, the GFP in a misfolded conformation can be secreted by Tat pathway in B. subtilis [13]. Further experiments should be conducted to confirm the secretion type of the Nsp. By the use of intrinsically disordered protein, it will be valuable to study the factors of mature proteins affecting the selected protein export pathway by YwbN and PhoD signal peptides.
In striking contrast to the classical secretory proteins, some proteins have been found to be secreted without any apparent signal peptide. Due to lack of proper research, the molecular mechanism that mediates this process and signals which directs export of these proteins are still unknown. Yang et al. demonstrated that release of the nonclassical proteins is not due to cell lysis. Furthermore, they identified a hydrophobic alpha-helical domain within enolase that contributes to its secretion, but this domain is not sufficient to promote the secretion of green fluorescent protein as a marker protein [21]. In this study, we found that Nsp can be secreted when fused to non-classical secreted proteins. As opposed to the case of Sec-type signal peptide, the fusion protein was detected in the cell fraction. Our results did not reveal the basis of fusion protein accumulation in the cell fraction. Perhaps the fusion protein forms protease-resistant conformation when bound to cytoplasmic targeting factors. Alternatively, it might form polymeric protein to resist the degradation. The appearance of Nsp in the supernatant required a function export signal, because Nsp expressed in cytoplasm was not detected. As Nsp was exported to the supernatant when fused to non-classical secretory protein, it is possible to use Nsp as the reporter to seek the export signal of non-classical secretory proteins.
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22:1399–1408
Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T (2006) Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in gram-positive bacteria. J Mol Biol 362:393–402
Brockmeier U, Wendorff M, Eggert T (2006) Versatile expression and secretion vectors for Bacillus subtilis. Curr Microbiol 52:143–148
Chen MW, Nagarajan V (1993) Chloramphenicol acetyltransferase, a cytoplasmic protein is incompatible for export from Bacillus subtilis. J Bacteriol 175:5697–5700
Cohen SN, Chang ACY, Hsu L (1972) Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA 69:2110–2114
Cooper DN, Barondes SH (1990) Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol 110:1681–1691
Jacobs MF, Andersen JB, Borchert TV, Kontinen VP (1995) Identification of a Bacillus subtilis secretion mutant using a beta-galactosidase screening procedure. Microbiology 141:1771–1779
Jongbloed JDH, Grieger U, Antelmann H, Hecker M, Nijland R, Bron S, van Dijl JM (2004) Two minimal Tat translocases in Bacillus. Mol Microbiol 54:1319–1325
Kolkman MAB, van der Ploeg R, Bertels M, van Dijk M, van der Laan J, van Dijl JM, Ferrari E (2008) The Twin-arginine signal peptide of Bacillus subtilis YwbN can direct either Tat- or Sec-dependent secretion of different cargo proteins: secretion of active subtilisin via the B. subtilis Tat pathway. Appl Enviro Microbiol 74(24)):7507–7513
Lee C, Li P, Inouye H, Brickman ER, Beckwith J (1989) Genetic studies on the inability of beta-galactosidase to be translocated across the Escherichia coli cytoplasmic membrane. J Bacteriol 171:4609–4616
Li WF, Zhou XX, Lu P (2004) Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res Microbiol 155:605–610
Meissner D, Vollstedt A, van Dijl JM, Freudl R (2007) Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different gram-positive bacteria. Appl Microbiol Biotechnol 76:633–642
Natale P, Brüser T, Driessen AJM (2008) Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane—distinct translocases and mechanisms. Biochim Biophys Acta 1778:1735–1756
Rubartelli A, Cozzolino F, Talio M, Sitia R (1990) A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J 9:1503–1510
Simonen M, Palva I (1993) Protein secretion in bacillus species. Microbiol Rev 57:109–137
Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM (2004) Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev 68:207–233
Vladimir N, Uversky A, Dunker K (2010) Understanding protein non-folding. Biochim Biophys Acta 1804:1231–1264
Xia Y, Zhao J, Chen H, Liu X, Wang Y, Tian F, Zhang HP, Zhang H, Chen W (2010) Extracellular secretion in Bacillus subtilis of a cytoplasmic thermostable β-galactosidase from Geobacillus stearothermophilus. J Dairy Sci 93:2838–2845
Yamada J, Phillips JL, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, Reza R, Acheson J, Krishnan VV, Newsam S, Gopinathan A, Lau EY, Colvin ME, Uversky VN, Rexach MF (2010) A bimodal distribution of two distinct categories of intrinsically disordered structures with separate functions in FG nucleoporins. Mol Cell Proteomics 9:2205–2224
Yang CK, Ewis HE, Zhang X, Lu CD, Hu HJ, Pan Y, Abdelal AT, Tai PC (2011) Nonclassical protein secretion by Bacillus subtilis in the stationary phase is not due to cell lysis. J Bacteriol 193:5607–5615
Acknowledgments
We thank Hamid Majeed for critical review of this manuscript. This work was supported by the National Science Fund for Distinguished Young Scholars (31125021), the National High Technology Research and Development Program of China (2011AA100905), the National Natural Science Foundation of China (No. 31171636), the Key program of National Natural Science Foundation of China (No. 20836003), the National Basic Research Program of China 973 Program (2012CB720802), the 111 project B07029, and SKLF-TS-201101.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, G., Chen, H., Zhang, H. et al. The Secretion of an Intrinsically Disordered Protein with Different Secretion Signals in Bacillus subtilis . Curr Microbiol 66, 566–572 (2013). https://doi.org/10.1007/s00284-013-0315-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0315-8