Abstract
Staphylococcus aureus is a leading cause of nosocomial infections due to its resistance to diverse antibiotics. This bacterium produces a large number of extracellular virulence factors that are closely associated with specific diseases. In this study, diverse plant flavonoids were investigated to identify a novel anti-virulence compound against two S. aureus strains. Flavone, a backbone compound of flavonoids, at subinhibitory concentration (50 μg/mL), markedly reduced the production of staphyloxanthin and α-hemolysin. This staphyloxanthin reduction rendered the S. aureus cells 100 times more vulnerable to hydrogen peroxide in the presence of flavone. In addition, flavone significantly decreased the hemolysis of human red blood by S. aureus, and the transcriptional level of α-hemolysin gene hla and a global regulator gene sae in S. aureus cells. This finding supported the usefulness of flavone as a potential antivirulence agent against antibiotic-resistant S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is an important human pathogen that often exhibits antibiotic resistance and is responsible for worldwide outbreaks of nosocomial infections [14]. This pathogen can secrete several exotoxins, such as hemolysin, enterotoxins, coagulase, TSST-1, and protein A, which are associated with specific diseases [16]. S. aureus strains are also capable of producing the golden carotenoid pigment, staphyloxanthin that acts as a virulence factor, primarily being a bacterial antioxidant which protects the pathogen from the host’s immune system in the form of reactive oxygen species [3, 13].
Over the past several decades, numerous antibiotics have been developed and used for bacterial infections. However, there has been a significant decrease in the rate of discovery of new antibiotics [13]. Furthermore, current usage of bactericidal compounds is often unsuccessful because of the emergence of methicillin-resistant S. aureus [2, 12]. Hence, unlike antibiotics that mostly aim to inhibit cell growth, alternative approaches such as antivirulence compounds have attracted strong research interest. The antivirulence approach aims to reduce the production of virulence factors without affecting bacterial growth to impede the possible emergence of drug resistance [2, 7].
Major discoveries in the antivirulence approach against S. aureus include the inhibition of (i) the virulence factor staphyloxanthin [13], (ii) enterotoxins and hemolysins [24] (iii) antibiotic resistant biofilm formation [1, 8, 9], and (iv) bacterial quorum sensing [18]. Recently, several plant compounds have been reported to decrease the virulence of S. aureus without affecting its growth. For example, thymol found in thyme [20] reduced enterotoxins and α-hemolysin production; luteolin [19] and chrysin [23] reduced α-hemolysin production; and fisetin [5] and olelic acid [22] inhibited the biofilm formation in S. aureus.
The overall aim of this study was to identify novel and potent antivirulence compounds from the screening of plant flavonoids against S. aureus. We investigated the effects of 12 flavonoids on the production of virulence factors, such as staphyloxanthin and α-hemolysin in S. aureus. Among the tested flavonoids, a subinhibitory concentration of flavone was identified as the most potent antivirulence compound without antimicrobial activity. This is the first article reporting on the use of flavone to reduce the production of both staphyloxanthin and α-hemolysin of S. aureus.
Materials and Methods
Bacterial Strains and Chemicals
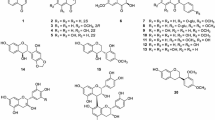
All experiments were conducted at 37 °C, and trypticase soy broth (TSB) was used for the cultures of S. aureus (ATCC 25923) and S. aureus (ATCC 6538), which were obtained from the Korean Agricultural Culture Collection. Two S. aureus strains were used to reinforce our findings. Chemicals including 12 flavonoids (flavone (99 %), 6-aminoflavone (97 %), 6-hydroxyflavone (98 %), apigenin (97 %), chrysin (97 %), curcumin (94 %), daidzein (98 %), fisetin (98 %), genistein (98 %), luteolin (98 %), phloretin (99 %), and quercetin (98 %)) were purchased from Sigma-Aldrich Co. (Missouri, USA). The structures of the flavonoids are shown (Fig. 1). All the 12 flavonoids were dissolved in dimethyl sulfoxide (DMSO).
Effect of flavonoids on the production of staphyloxanthin in S. aureus. S. aureus (ATCC 25923) was used. Cell pellets of 16-h grown S. aureus with or without flavonoids were observed for the production of staphyloxanthin. Fla flavone, 6AF 6-aminoflavone, 6HF: 6-hydroxyflavone, Chr chrysin, Api apigenin, Phl phloretin, Fis fisetin, Lut luteolin, Que quercetin, Dai daidzein, Gen genistein, and Cur curcumin. All flavonoids were used at 50 μg/mL, except luteolin, which was used at 25 μg/mL because of its antimicrobial activity. All the compounds were dissolved in DMSO. DMSO was used as a control. The structures of the flavonoids are shown. The experiment was done in triplicate, and representative images are shown
Bacterial Culture
Staphylococcus aureus strains were initially streaked from −80 °C glycerol stock on LB plates, and a fresh single colony was inoculated in TSB (2 mL) in 14-mL tubes and cultured at 37 °C and 250 rpm for all experiments except cell growth measurement. Overnight cultures were re-inoculated at 1:100 dilution in the medium. For cell growth measurements, a fresh single colony was inoculated in TSB (25 mL) contained in 250-mL flasks, and cultured at 37 °C and 250 rpm, and optical densities were measured at 600 nm using a spectrophotometer (UV-160, Shimadzu, Japan). Each experiment was performed with at least two independent cultures.
Staphyloxanthin Assay
The bright golden coloration of this virulence factor facilitates the anti-virulence screening by the simple observation of color change [6]. Also, a quantitative carotenoid assay method was adapted from the previous method [15]. In brief, cells were re-inoculated at 1:100 dilution in TSB medium and incubated for 16 h at 37 °C with or without flavonoids. Cells (1 mL) were then collected by centrifugation at 16,600×g for 1 min and washed with 1 ml of phosphate-buffered saline (PBS). At this point, cell pellets were photographed to compare the staphyloxanthin production. For the extraction of carotenoid pigments, the cell pellets were resuspended in 0.2 mL of methanol by vortexing, and this mixture was heated at 55 °C for 3 min. Pigment extraction was separated from cell debris by centrifugation at 16,600×g for 10 min. This pigment extraction step was repeated 3 times, and the optical densities of collected extractions were measured at 465 nm using a spectrophotometer (UV-160, Shimadzu, Japan). Each data point was averaged from at least three independent cultures.
Hydrogen Peroxide Resistance Assays
The resistance assay (survival test) with hydrogen peroxide was adapted from previous study [13]. Overnight cultures grown for 16 h in TSB were re-grown to mid-log phase in TSB (turbidity at 600 nm of 1). Then, 0.1 mL of each culture was incubated with H2O2 at a final concentration of 1.5 % (v/v) for 60 min with shaking at 250 rpm. The percentage of cells surviving the stresses was calculated as the number of colony-forming units (CFU)/mL remaining after each stress divided by the initial CFU/mL. Three independent experiments were conducted.
Hemolysis Assay
Hemolysis analysis was modified from the previous method [10]. The lysis efficacy of human red blood cells was measured with whole cultures of S. aureus grown in the presence of flavonoids. In brief, S. aureus cells were diluted at 1:100 with an overnight culture in TSB and cultured with or without all flavonoids at 50 μg/mL except luteolin at 25 μg/mL at 37 °C for 16 h with shaking at 250 rpm. The cell cultures (50 μL including cells and culture supernatant) were added into diluted human red blood cells that had previously been separated by centrifugation at 900×g for 5 min, washed with PBS buffer three times and diluted at 3 % of red blood cells in PBS buffer. For hemolytic activity, the mixture was incubated at 37 °C for 1 h with 250 rpm shaking. The supernatant was collected by centrifugation at 16,600×g for 10 min, and the optical density was measured at 543 nm.
RNA Isolation and Real-time qRT-PCR
Staphylococcus aureus (ATCC 25923) was cultivated in TSB with or without flavone for 16 h at 250 rpm. Before taking samples, RNase inhibitor (RNAlater, Ambion, TX, USA) was added, and cells were immediately chilled for 30 s with dry ice and 95 % ethanol (to prevent RNA degradation) before centrifugation at 13,000×g for 2 min. The cell pellets were immediately frozen with dry ice and stored at −80 °C. Total RNA was isolated using a Qiagen RNeasy mini Kit (Valencia, CA, USA). To remove all DNA, the purified RNA was treated for 15 min with 30 Units of DNase I. To investigate the transcriptions of hla (α-hemolysin gene), sae (a global regulator), agrA (quorum-sensing gene), sar (accessory regulator A), sigB (RNA polymerase sigma factor), and seo (enterotoxin), they were quantified using qRT-PCR. The primer pairs for qRT-PCR are presented in Table 1. The 16S rRNA housekeeping gene was used. Real-time qRT-PCR was performed using the StepOneTM Real-Time PCR system (Applied Biosystems, Foster City, CA) and SuperScriptTM III Platinum® SYBR® Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA).
Results
Investigation of Flavonoids for Staphyloxanthin Reduction in S. aureus
To investigate the antivirulence activity against S. aureus, (ATCC 25923), 12 flavonoids at subinhibitory concentrations (10, 25, and 50 μg/mL) were screened for the reduction of staphyloxanthin production. The golden pigment staphyloxanthin could be visually identified in the cell pellets of S. aureus. Among the tested flavonoids, flavone, which is the backbone compound of flavonoids, most significantly reduced the staphyloxanthin production in S. aureus (Fig. 1). Quantitative analysis also clearly indicated that flavone reduced the staphyloxanthin production by tenfold compared with non-treatment control (data not shown). In the case of luteolin, its antimicrobial activity at 25 μg/mL [19] reduced the cell growth and staphyloxanthin production. The result of staphyloxanthin inhibition by flavone was similar to those in another S. aureus strain (ATCC 6538) (data not shown).
Flavone Reduced Hydrogen Peroxide Resistance
Staphyloxanthin acts as an antioxidant by enabling the detoxification of host-immune system-generated ROS such as oxygen radical (O2−) and hydrogen peroxide (H2O2) [13]. Hence, we examined the effect of flavone on the survival rate of S. aureus in the presence of H2O2. As expected, flavone reduced H2O2 susceptibility by 100-fold, while structurally similar chrysin and 6-hydroxyflavone had no or much less effect on the survival rate (Fig. 2). This result was attributed to the effect of flavone in reducing staphyloxanthin production in S. aureus.
Effect of flavonoids on hydrogen peroxide resistance. The survival of S. aureus (ATCC 25923) with or without flavonoids (chrysin, 6-hydroxyflavone (6HF), and flavone) was measured after H2O2 (1.5 %, v/v) treatment for 60 min. Flavonoids were used at 50 μg/mL. The percentage of survive cells was calculated as the number of colony forming units (CFU) per ml remaining after the H2O2 stress divided by the initial CFU per ml. The experiment was performed in triplicate
Flavone Reduced Hemolysis by S. aureus without Affecting the Growth of Planktonic Cells
As S. aureus can produce α-hemolysin, which is a pore-forming cytotoxin and causes hemolysis, we investigated the effect of flavonoids on blood hemolysis by S. aureus. Among the 12 tested flavonoids, eight showed a significant antihemolytic activity (Fig. 3). This result is consistent with that of the previous study [19] in that luteolin at subinhibitory concentration abolished the hemolysis activity of S. aureus. Moreover, seven more flavonoids, flavone, 6-aminoflavone, 6-hydroxyflavone, apigenin, phloretin, fisetin, and genistein, also markedly reduced hemolysis activity at their subinhibitory concentrations. Particularly, flavone clearly and dose-dependently inhibited the hemolytic activity of two S. aureus strains after 16-h culture (Fig. 4a, b). The reduction of hemolytic activity by flavone was similar at the different culture-time points, such as 12 and 24 h (data not shown).
Effect of flavonoids on hemolysis. The hemolysis screening was performed using human red blood cells upon adding S. aureus (ATCC 25923) cultures (50 μL) grown with flavonoids for 16 h. Fla flavone, 6AF 6-aminoflavone, 6HF 6-hydroxyflavone, Chr chrysin, Api apigenin, Phl phloretin, Fis fisetin, Lut luteolin, Que quercetin, Dai daidzein, Gen genistein, and Cur curcumin. All flavonoids were used at 50 μg/mL, except luteolin, which was used at 25 μg/mL because of its antimicrobial activity
Effect of flavone on hemolysis and cell growth in two S. aureus strains. The hemolysis assay was performed using human red blood cells upon adding two S. aureus (ATCC 25923 and ATCC 6538) cultures (50 μL) grown with flavone (0, 25, and 50 μg/mL) for 16 h. Pictures of the spectrophotometer cuvettes are shown for the hemolysis activity. Planktonic cell growth of S. aureus was measured at 600 nm in 250 mL-flasks with 250 rpm
A potential antivirulence compound without antimicrobial activity is preferred as this avoids the possible development of bacterial drug resistance. Thus, the toxicity of flavone was investigated by measuring the growth of planktonic S. aureus cells. Although flavone at 50 μg/mL slightly delayed the cell growth of two S. aureus strains, the growth was recovered at 14 h (Fig. 4c, d). In addition, the specific growth rates have been measured. In the absence of flavone, the specific growth rates of S. aureus ATCC 25923 and ATCC 6538 were 1.35 ± 0.18/h and 1.23 ± 0.21/h, whereas the growth rates were 1.05 ± 0.12/h and 1.22 ± 0.21/h with flavone at 50 μg/mL, respectively. Furthermore, the numbers of viable cells were not significantly affected by flavone at 50 μg/mL (data not shown). The overall growth data indicated that the reduction of staphyloxanthin and antihemolytic activity of flavone was due to its antivirulence activity rather than antimicrobial activity.
Flavone Repressed the Transcription of α-Hemolysin
To investigate the mechanism of flavone’s antivirulence activity, real-time qRT-PCR was used to determine a differential expression of virulence factor-related genes, such as hla (α-hemolysin gene), sae (a global regulator inducing hla [17], and agrA (quorum-sensing gene), in S. aureus cells with and without flavone. Flavone clearly repressed the transcription of hla by 11-fold and sae by fourfold (Fig. 5), which supports the reduction of hemolysis in S. aureus cells by flavone (Fig. 4). However, flavone elevated agrA transcription by fourfold and did not change the transcription of other virulence factor genes such as sar, sigB and seo (Fig. 5). The results support the previous finding that the agr and sae might be inhibiting each other [17].
Transcriptional profiles of S. aureus cells in the presence of flavone. Flavone was used at 50 μg/mL. Transcriptional profiles were measured by qRT-PCR. Fold change represents the change (n-fold) in transcription compared to the data in the absence of flavones (white bars, value of 1.0). The experiment was performed in duplicate
Discussion
In this study, we utilized dual screening to inhibit various virulence factors, such as staphyloxanthin and α-hemolysin, in S. aureus. Among 12 plant flavonoids, flavone reduced the production of staphyloxanthin, H2O2 resistance, and blood hemolysis without inhibiting the planktonic growth of S. aureus. This article is noteworthy as it is the first one to report on the use of flavone to reduce both the hemolytic ability and staphyloxanthin production of S. aureus (Figs. 1, 3, and 4).
Flavonoids are ubiquitous in plants and are commonly found in fruit, vegetables, nuts, seeds, stems, and flowers. They are biologically active in combating diseases in humans because of their diverse biological functions, such as antioxidative, antifungal, antiviral, antibacterial, and anticarcinogenic activities [4]. As the daily dietary intake of mixed flavonoids is estimated to be in the range of 500–1,000 mg [21], they are likely to have minimal toxicity to humans [4], but further study is warranted to confirm this. Recently, the flavonoids luteolin [19] and chrysin [23] at subinhibitory concentrations showed an ability to inhibit the hemolysis of S. aureus, and fisetin reduced the antibiotic-resistant biofilm formation in S. aureus [5], which demonstrated the potential antivirulence activity of these flavonoids. Compared with luteolin, chrysin, and fisetin, flavone specifically reduced the virulence factor of staphyloxanthin and the H2O2 resistance. Therefore, the present results have expanded the scope of previous studies and demonstrated that the functional groups of flavonoids differentially control several virulent phenotypes of S. aureus. Flavone is the simplest form among flavonoids used in this study. Although it is speculative, only this simple flavone can be easily transported into S. aureus cells and bound to regulatory proteins, while other larger flavonoids may have a transport problem into cells and have a less binding affinity to some regulatory proteins. Further investigation is required to understand how flavone rather than other larger analogs specifically works in S. aureus cells.
The expansion in bacterial resistance to antibiotics has created an urgent need for effective antimicrobial agents as well as antivirulence compounds against pathogenic bacteria. In this study, the dual screening of 12 flavonoids for two virulence factors was performed against S. aureus, and flavone demonstrated potential as a new potent antivirulence compound. Although the exact action mechanisms of flavone’s antivirulence activity remains to be determined, the results suggest that the screening of a larger library of flavonoids will generate more potent therapeutics for the human pathogen S. aureus, and possibly for other pathogens as well. Recently, the flavonoid phloretin, which is abundant in apples, reduced the attachment of Escherichia coli O157:H7 to human colonic epithelial cells and also diminished colon inflammation in a rat model [11]. Therefore, natural flavonoids are important sources for antivirulence compounds and flavone can be used as a basic structure in the design of potent antivirulence drugs.
References
Boles BR, Horswill AR (2011) Staphylococcal biofilm disassembly. Trends Microbiol 19:449–455
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6:17–27
Clauditz A, Resch A, Wieland KP, Peschel A, Götz F (2006) Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74:4950–4953
Cushnie TP, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356
Dürig A, Kouskoumvekaki I, Vejborg RM, Klemm P (2010) Chemoinformatics-assisted development of new anti-biofilm compounds. Appl Microbiol Biotechnol 87:309–317
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Hoiby N, Kjelleberg S, Givskov M (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87–102
Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-amino acids trigger biofilm disassembly. Science 328:627–629
Larzabal M, Mercado EC, Vilte DA, Salazar-Gonzalez H, Cataldi A, Navarro-Garcia F (2010) Designed coiled-coil peptides inhibit the type three secretion system of enteropathogenic Escherichia coli. PLoS One 5:e9046
Lee JH, Regmi SC, Kim JA, Cho MH, Yun H, Lee CS, Lee J (2011) Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect Immun 79:4819–4827
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129
Liu CI, Liu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, Oldfield E (2008) A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 319:1391–1394
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Morikawa K, Maruyama A, Inose Y, Higashide M, Hayashi H, Ohta T (2001) Overexpression of sigma factor, σB, urges Staphylococcus aureus to thicken the cell wall and to resist β-lactams. Biochem Biophys Res Commun 288:385–389
Ohlsen K, Koller KP, Hacker J (1997) Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla:lacZ gene fusion. Infect Immun 65:3606–3614
Pantrangi M, Singh VK, Wolz C, Shukla SK (2010) Staphylococcal superantigen-like genes, ssl5 and ssl8, are positively regulated by Sae and negatively by Agr in the Newman strain. FEMS Microbiol Lett 308:175–184
Park J, Jagasia R, Kaufmann GF, Mathison JC, Ruiz DI, Moss JA, Meijler MM, Ulevitch RJ, Janda KD (2007) Infection control by antibody disruption of bacterial quorum sensing signaling. Chem Biol 14:1119–1127
Qiu J, Li H, Meng H, Hu C, Li J, Luo M, Dong J, Wang X, Wang J, Deng Y, Deng X (2011) Impact of luteolin on the production of alpha-toxin by Staphylococcus aureus. Lett Appl Microbiol 53:238–243
Qiu J, Wang D, Xiang H, Feng H, Jiang Y, Xia L, Dong J, Lu J, Yu L, Deng X (2010) Subinhibitory concentrations of thymol reduce enterotoxins A and B and α-hemolysin production in Staphylococcus aureus isolates. PLoS One 5:e9736
Skibola CF, Smith MT (2000) Potential health impacts of excessive flavonoid intake. Free Radic Biol Med 29:375–383
Stenz L, Francois P, Fischer A, Huyghe A, Tangomo M, Hernandez D, Cassat J, Linder P, Schrenzel J (2008) Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. FEMS Microbiol Lett 287:149–155
Wang J, Qiu J, Dong J, Li H, Luo M, Dai X, Zhang Y, Leng B, Niu X, Zhao S, Deng X (2011) Chrysin protects mice from Staphylococcus aureus pneumonia. J Appl Microbiol 111:1551–1558
Wyatt MA, Wang W, Roux CM, Beasley FC, Heinrichs DE, Dunman PM, Magarvey NA (2010) Staphylococcus aureus nonribosomal peptide secondary metabolites regulate virulencev. Science 329:294–296
Acknowledgments
This research was supported by the Yeungnam University Research Grant and Bio-industry Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin-Hyung Lee and Joo-Hyeon Park contributed equally to this study
Rights and permissions
About this article
Cite this article
Lee, JH., Park, JH., Cho, M.H. et al. Flavone Reduces the Production of Virulence Factors, Staphyloxanthin and α-Hemolysin, in Staphylococcus aureus . Curr Microbiol 65, 726–732 (2012). https://doi.org/10.1007/s00284-012-0229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0229-x