Abstract
Root colonization and diversity of arbuscular mycorrhizal fungi (AMF) were analyzed in plants growing in fly ash pond. Eight species could be separated morphologically, while phylogenetic analyses after PCR amplification of the ITS region followed by RFLP and sequencing revealed seven different AM fungal sequence types. Phylogenetic analysis showed that these sequences cluster into four discrete groups, belonging to the genus Glomus and Archaeospora. Inoculation of plants with spores of AM fungal consortia (Glomus etunicatum, Glomus heterogama, Glomus maculosum, Glomus magnicaule, Glomus multicaule, Glomus rosea, Scutellospora heterogama, and Scutellospora nigra) along with colonized root pieces increased the growth (84.9%), chlorophyll (54%), and total P content (44.3%) of Eucalyptus tereticornis seedlings grown on fly ash compared to non-inoculated seedlings. The growth improvement was the consequence of increased P nutrition and decreased Al, Fe, Zn, and Cu accumulations. These observations suggested that the inoculation of tree seedlings with stress adapted AM fungi aid in the reclamation of fly ash ponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash disposal is a major environmental concern throughout the coal-based power generating countries. In India alone, the annual production of fly ash is about 120 MT produced by 82 power plants and is expected to rise to 150–170 MT per year by the end of 2012 [24]. Disposal of fly ash has significant impact on terrestrial and aquatic ecosystems due to leaching of toxic substances from the ash into soil and ground water, as well as reduction in plant establishment and growth [18, 51, 52]. In many cases, the reclamation practices have been ineffective in reducing mortality of tree seedlings due to deficiency of essential nutrients (usually N and P), low soil microbial activity, high soluble salt concentrations of trace elements, and the presence of compacted and cement layers on ash disposal sites [41]. The lack of mycorrhizal symbionts, which enhance nutrient supply to the host plants and alleviate biotic stress, may be partly responsible for the problems in revegetation [43]. Mycorrhizal fungi are a ubiquitous group of soil microorganisms that form symbiotic association with roots of the majority of terrestrial plant species [42]. Arbuscular mycorrhizal fungi (AMF) are considered to be ecologically important as they play important roles in the restoration of contaminated ecosystems by improving plant nutrition and fertility of degraded land [7].
Spontaneous selection of infective and effective AM fungi can be a long process in fly ash ponds. Application of selected metal tolerant AMF strains may increase the rate of restoration, efficiency of phytoremediation and speeds up these processes [47]. Isolation and mass inoculum of AM fungi that are adapted to fly ash may have more beneficial effect as compared to other AM fungi. It has also been demonstrated that the use of adapted AM fungal strains can be more effective in restoration and bioremediation studies than the non-adapted strains [46]. Molecular and biochemical basis of well-adapted AM fungal strains for restoration and bioremediation correlated with their origin from polluted sites is still not clear. Hence, it is necessary to understand the ecological role of AM fungi associated with plants growing in fly ash. Selvam and Mahadevan [41] studied the distribution of AM fungal species naturally associated with plants in an abandoned lignite fly ash pond. Amendment of farm yard manure to fly ash along with AM fungi improved the growth, physical properties of fly ash, and reduction of toxic metals [19]. Several researchers have reported the effects of selected isolates of AM fungi on plant growth, nutrient uptake, and aggregation of fly ash [11, 52]. The morphological methods may not reflect the actual diversity and functionally active AM fungi associated with plant roots [8]. It has hitherto been difficult to identify each individual fungal species that inhabits the root of a plant. Alternatively molecular approaches have been shown to hold great promise in estimating biodiversity and population structure of AM fungi [39]. Molecular identification of AM fungi based on the sequences of ITS and LSU of rDNA has been widely used to characterize AM fungal communities under native forests, grasslands, agriculture ecosystems, and contaminated soils [13, 21, 36, 37].
In this investigation, we have determined the colonization levels and the diversity of AM fungi associated with different plants growing on a fly ash pond using both spore morphology and molecular characterization. An attempt has also been made to understand the effect of these AM fungi on the growth of Eucalyptus tereticornis seedlings grown on fly ash.
Materials and Methods
Study Site
Fly ash pond located at National Aluminium Company Ltd. (NALCO), Damnjodi (18°46′22″N, 082°53′23″E), Orissa, India, was selected for this study. The fly ash chemical properties are represented in Table 1. The roots and the surrounding fly ash rhizosphere samples were collected from six dominant plant species, Acacia pennata (Mimosaceae), Calotropis gigantea (Asclepiadaceae), Cassia occidentalis (Caesalpiniaceae), Cynodon dactylon (Poaceae), Lantana camara (Verbenaceae), and Jatropha gossypifolia (Euphorbiaceae) growing in the pond in the month of April 2007 (summer season). Ten plants each of above mentioned species were randomly selected, and about 500 g of rhizosphere sample of each plant was collected at a depth of 0–30 cm. Root and soil samples representative for each plant species were pooled separately to yield one composite sample. The samples were placed in polythene bags and stored at 5°C until processed.
AM Colonization and Spore Morphological Assessment
The percent root length colonization of AM fungi was calculated by the gridline intersect method [15] after staining with trypan blue [33]. AM fungal spores were isolated from rhizospheric fly ash samples by wet sieving and decanting technique [14]. The spores were identified following current taxonomic criteria [40] and also using information of INVAM (http://www.invam.caf.wdu.edu/).
DNA Extraction and PCR Amplification
Fine root samples collected from fly ash pond were pooled and ground to powder in liquid nitrogen. Five plants of each species colonized with AM fungi were used for DNA extraction. Genomic DNA was extracted using DNeasy Plant Mini Kit following the manufacturer’s instructions (Qiagen, Hilden, Germany). Nested PCR was performed for amplification of partial 18S-ITS1-5.8S-ITS2 rDNA region of AM fungi as described by Redecker [35], with minor modification. The first round of amplification was performed using NS5 and ITS4 primers [50] with an annealing temperature of 51°C. For the second amplification, PCR amplicons were diluted in TE buffer (1:10, 1:50, and 1:100), and PCR was performed using genus specific primers ARCH1311, ACAU1660, LETC1670, GLOM1310, PARA1313, in combination with ITS4i in separate reactions [36]. The reverse primer GLOM5.8 was used only in combination with NS5 primer [36]. The 25-μl reaction mixture consisted of 1× PCR buffer, 1.5 mM MgCl2, 200 μM of each dNTPs, 0.5 μM of each primer, and 0.15 units of Taq DNA polymerase (Larova, Teltow, Germany). The PCR conditions were as follows: initial denaturation at 95°C for 4 min, followed by 25 cycles of 1 min at 95°C, 1 min at 61°C and 1 min at 72°C, and a final extension of 72°C for 8 min.
Cloning and Sequence Analyses
PCR products were run on 1.5% agarose gel, and the target bands were excised and purified with QIAquick PCR purification Kit (Qiagen, USA) following the manufacture’s recommendations. The purified products were cloned separately into pTZ57R/T vector of the InsTAclone™ PCR Cloning Kit (Fermentas) and transformed into competent Escherichia coli DH5α. The plasmid DNA was isolated from 150 randomly selected transformants, and inserts were re-amplified with specific genus primers. RFLP analysis was performed using HinfI and MboI enzymes as per manufacturer’s recommendations and run on a 1.8% agarose gels. The clones of distinct RFLP patterns were sequenced using Applied Biosystems DNA sequencer. Sequence similarities were determined using the BLAST analysis of NCBI. Sequences of AM fungi were deposited at NCBI database under the accession numbers HM159456 to HM159462. The sequences were aligned using MAFFT v 6.240 [20] with other sequences obtained from GenBank. The phylogenetic analysis was carried out by neighbor-joining method using MEGA software [45] with Kimura 2-parameter model. Bootstrap values of 1000 were used to assess the relative support for each clade.
Nursery Experiment
The inoculum of AM fungi was prepared in earthen pots by isolating the spores from the rhizosphere of fly ash and root pieces. The inoculum was propagated in fly ash using maize, wheat, and sorghum as trap plants. No fertilizers were applied while multiplying the AM fungi. The pots were watered with tap water and maintained under green house conditions. The inoculum was prepared with density of 450–500 spores per 10 g of fly ash along with AM-infected root pieces. The majority of the spores found in the inoculum were Glomus rosea, Glomus etunicatum, Glomus heterogama, Glomus maculosum, Glomus magnicaule, Glomus multicaule, Scutellospora heterogama, and Scutellospora nigra. Seeds of E. tereticornis were surface sterilized and sown in trays containing heat sterilized (121°C for 3 h) sand:soil (1:1 w/w) mixture. Two-week-old seedlings with similar height (5 cm) were used for plantation. The nursery experiment consisted of two treatments: control (fly ash alone) and inoculated treatments (fly ash + AM fungi) with 15 replicates each. Fly ash was filled in polythene bags (1.5 kg capacity). The inoculum (10 g) was mixed with fly ash and transplanted one seedling per bag. Seedlings were watered regularly and allowed to grow for 6 months without adding any fertilizer. Plants were harvested after 6 months and analyzed for various parameters.
Plant Growth Parameters and AM Fungal Colonization
The height of the plants and dry biomass of shoots and roots were determined. Chlorophyll content was estimated after extraction in 80% chilled acetone according to Arnon [2]. The percent root length colonization of AM fungi was assessed. The dried and ground shoot material of E. tereticornis seedlings were digested with the mixture of concentrated HNO3/HClO4 (3:1 v/v) until the contents become colorless and only white dense fumes appeared. It was cooled, and diluted HCl was added [31]. The concentration of different metals (Al, Fe, Zn, and Cu) was measured in the filtrate by Inductively Coupled Plasma Mass Spectroscopy.
Physico-chemical and Biochemical Analysis of Fly Ash
The pH and electric conductivity (EC) of fly ash was determined. Organic carbon was estimated according to the method described by Walkley and Black [49]. The total N content was determined by total organic carbon (TOC) analyzer (Thermo-Hyper TOC, Thermo scientific, USA) coupled with total nitrogen module. Available P was estimated by Bray and Kurtiz [6] method. Urease activity was determined by the method of McGarity and Myers [23], and the assay of acid phosphatase activity was determined by measuring the p-nitrophenol released by phosphatase activity [44].
Statistical Analysis
The data were statistically analyzed, and the means were compared using t test at P < 0.05. The statistical analyses were performed using GraphPad prism software v.4.03.
Results
Arbuscular Mycorrhizal fungal colonization was observed in root samples of all the plants growing in fly ash pond. Average percent colonization of AM fungi varied from 14 to 33%. Maximum colonization was observed in J. gossypifolia, while the minimum in C. dactylon (Table 2). Spores of AM fungi were very low in rhizosphere soil samples of each plant species and varied from 8 to 40 spores/kg (Table 2). The higher number of spores was counted in the rhizosphere soil of A. pennata (Table 2). Spores isolated from fly ash pond were identified based on their morphology. Eight different AM fungal morphotypes were observed, and among these, six were identified as Glomus (G. etunicatum, G. heterogama, G. maculosum, G. magnicaule, G. multicaule, and G. rosea) and two as Scutellospora (S. heterogama and S. nigra). G. rosea was the dominant fungus from these soils. Among the six plant species, Lantana camara displayed all identified AM fungal species except G. magnicaule compared to other plants. AM fungal species and their occurrence for different plant species growing in ash pond are listed in Table 2.
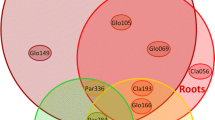
Nested PCR results revealed that only GLOM1310/ITS4i and ARCH1311/ITS4i primers amplified the target DNA. After cloning of amplified products, a total of 44 RFLP types were detected. BLAST results of 18S-ITS1-5.8S-ITS2 rDNA region sequences indicated that among 44 cloned sequences, 7 clones (FA8, FA14, FA19, FA23, FA29, FA31, and FArc1) showed similarity to Glomeromycota and the other sequences either Ascomycetes or Basidiomycetes. Of these seven sequences, five sequences showed homology with the genus Glomus (FA8, FA19, FA 23, FA29, and FA31) and one to Archaeospora (FArc1). The FA14 sequence did not show homology with known sequences. Phylogenetic analysis revealed that these sequences were clustered into four groups; two groups (Group I and II) belonging to genus Glomus, group III showing similarity with Archaeospora and group IV clustered without any reference sequences from the database. The sequences of FA19, FA23, and FA29 were clustered with G. irregular, G. intraradices, and some unidentified species of Glomus in group I. The FA31 clustered with G. indicum and FA8 with some unidentified species of Glomus in group II. Group III included sequence of FArc1 and was distantly associated with genus Archaeospora. The sequence of FA14 formed separate group (group IV) without any known sequences in the database (Fig. 1). The observations suggested that a limited number of AM fungi are associated with the plants growing in fly ash pond.
Neighbor-joining phylogenetic analysis of AM fungal partial 18S-ITS1-5.8S-ITS2 rDNA region sequences obtained from plant root samples of fly ash pond. Sequences obtained in the study are shown in boldface. Numerical values on branches are the bootstrap values as percentage of bootstrap replication from 1000 replicate analysis. The scale represents substitution per site
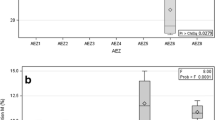
After 6 months, AM fungal colonization was observed in both inoculated and non-inoculated treatments. However, colonization of AM fungi in the inoculated treatments was four times more than the control treatment. AM colonization in control treatment may be due to the presence of some of the indigenous spores present in fly ash. The nature of AM fungal spores in control treatment was similar to some of the species (mostly Glomus spp.) occurring in fly ash. AM fungal colonization significantly increased the growth of E. tereticornis compared to the control treatment (Table 3). Inoculation of AM fungi increased the chlorophyll content in seedlings (55.5%) as compared to the control plants (Table 3). The AM fungal colonization significantly increased P concentration in the shoot of E. tereticornis in comparison with the non-inoculated treatment (Table 3). Uptake of Al, Zn, and Cu was significantly reduced in plants inoculated with AM fungi, but no significant difference in Fe uptake was observed (Table 3).
Chemical and biochemical properties of fly ash were positively affected by inoculum of AM fungi compared to initial or non-inoculated fly ash. Inoculation of AM fungi significantly increased the organic carbon (%), total N, and enzyme activities such as acid phosphatase and urease in fly ash compared to control treatment (Table 3). EC of fly ash was significantly high in both inoculated and non-inoculated treatments compared to initial value, and the maximum was recorded in AM inoculated fly ash (Table 3).
Discussion
The low rate of AM fungal colonization and the presence of very few spores in rhizosphere of plants grown on fly ash were attributed to adverse conditions. The soil structure and composition not only affect the spore population but also the biological activity of endophytes [26]. However, the presence of colonization and spores reflects the mycotrophic nature of the plant species studied and the ability of AM fungi to associate a wide range of host species in fly ash pond. In this study, it was reported that G. rosea had widest host range as it colonized most of the plant species. Variation in spore density/diversity and colonization of AM fungi associated with different host plant species may depend on a variety of mechanisms, including variation in host species and their phenology, mycorrhizal dependency, host plant-mediated alteration of the soil micro environment, or other unknown host plant traits [12, 22].
The identification of AM fungi using spore morphology and molecular approaches revealed that most species belong to the genus Glomus. This is consistent with the results of Selvam and Mahadevan [41] and Bedini et al. [5], who had reported Glomus as the most common species in fly ash. In this study, the species identified based on molecular characterization were not similar to those identified by spore morphology. This can be attributed to the fact that spore production might not have occurred in AM fungi associated with roots or some of these species may rely on vegetative strategies for colonization and dispersal [8]. On other hand, fungal spore diversity differs seasonally, with some fungi sporulating in late spring and others sporulating at the end of summer [34, 38]. Contrary to this, Guadarrama and Alvarez-Sanchez [17] reported that seasonality does not affect the abundance and richness of mycorrhizal spores. In this investigation, sampling was conducted in April (early summer) which may explain the presence of more AM fungal species detected by spore morphology versus molecular tools from roots. As spores represent the dormant state of the fungus, the physiologically active state is most likely the mirror image of the seasonal spore counts [38]. This factor can also affect spore number estimation as well as species diversity. In general, AM fungal identification is usually performed on the basis of specific spore morphological characteristics. However, the task of identifying spores based on their morphology is notoriously difficult due to alterations of morphological features during spore ontogeny or by parasitism [25, 48]. Therefore, combining molecular techniques with AM fungal morphotype assessment has enabled us to make detailed identifications of root-associated AM fungi in fly ash pond.
To the best of our knowledge, this is the first report on AM fungal molecular diversity in fly ash pond of electrostatic precipitator, although the molecular diversity of AM fungi had been reported from the bottom ash dump by Bedini et al. [5]. The primers GLOM1310 and ARCH1311 used in this study also amplified the sequences of Ascomycota and to a small extent Basidiomycota-related species (data not shown) due to nonspecific annealing of these primers. A similar observation was also reported by others [1, 35]. Phylogenetic analysis revealed that the sequences clustered into four discrete clades, and only a small portion of molecular clade was assigned to the known species including G. intraradices and G. irregular, leaving other sequences unidentified. Pennisi [32] emphasized on the growing number of unidentified fungal sequences without corresponding morphospecies records in the International Nucleotide Sequence Database Collaboration (INSDC) stating that the fungal diversity is higher than its current estimate. Our phylogenetic results support the hypothesis of Öpik et al. [29] that AM fungi may exhibit different distribution patterns, and a number of AM fungi related to G. intraradices may have global distribution. The AM fungi identified through spore morphology such as G. etunicatum, S. heterogama, and G. intraradices reported in this investigation have also been found in heavy metal contaminated soils [16, 53], suggesting their adaptability to different stress conditions.
The establishment of vegetation on ash pond using suitable microorganisms is a useful strategy in environmental management. Till date, rehabilitation performance has been found to be variable in many studies, and very little information is available on the effect of microorganisms on the vegetation cover on fly ash pond. In this investigation, the growth of E. tereticornis seedlings was significantly increased in AM inoculated plants as compared to control seedlings. It is also evident that AM fungi promote plant growth and increase soil aggregation [11, 52]. The AM fungi quickly lose their symbiotic efficacy when cultivated without edaphic stresses of the environment from where they have been originally isolated [10, 28]. It is also known that the inoculum should be produced with original edaphic stresses especially for AM isolates from extreme environments [28]. The AM fungal colonization increased in the inoculated treatment due to higher amount of inoculum and their adaptation to fly ash stress, and these were able to form mycorrhizae with E. tereticornis seedlings. The presence of AM fungal colonization in non-inoculated plants may be due to low number of propagules in fly ash and limited AM fungal diversity that results in low rate of colonization. The nature of spores present in the control treatment was similar to some of the species especially Glomus spp. present in the fly ash, because the fly ash used for nursery experiment was collected from the same site where we studied the diversity of AM fungi.
Although, fly ash contains many essential elements for plant uptake, non-essential elements such as Al, Fe, Zn, and Cu and many other elements might impair with various metabolic processes and so either delay or inhibit the process. It has also been reported that heavy metals decreased P concentration in shoots of E. globulus in absence of AM fungi [3]. Although plants of E. tereticornis accumulated various amounts of Al, Fe, Zn, and Cu in both treatments, the level of these metals was less when inoculated with AM fungi. The AM fungi increased the tolerance of plants by enhancing plant growth with a resulting dilution effect of metal in host plant or binding of the metal to the fungal cell wall through chitin or glomalin and immobilization in the rhizosphere or the roots [7, 16]. It has also been found that AM fungi alleviate metal toxicity especially Al, Fe, and Mn and enhance plant growth [27]. The production of chlorophyll was significantly reduced when plants were grown in contaminated soil [30]. However, total chlorophyll production increased in E. tereticornis plants significantly in the presence of AM fungal inoculum. The increased chlorophyll content of AM plants may results in increased photosynthesis efficiency of plants.
The chemical and biochemical properties of fly ash were improved by various degrees in inoculated treatments as compared to initial values. The pH of fly ash significantly reduced from slight alkaline to moderate acidic range in both treatments. The reduction of pH in fly ash may be due to the presence of sulfur content in fly ash while leaching of base ions [19]. Further, reduced pH levels in AM fungal inoculated fly ash samples may be attributed to the fact that AM fungi influenced organic acid exudation by plants resulting in reduced pH levels [9]. The AM fungi interact with other soil rhizosphere microorganisms and affect rhizodeposition, and thus the quality and quantity of organic C delivered to soil via fungal hyphae [4]. In general, enzymes are bio-chemical indicators of microbes in heavy metal contaminated soil. High enzymatic activity indicates improvement of contaminated soil properties as well as microbial community development. Among soil enzymes, acid phosphatase and urease play an important role in P, N, and C mineralization, respectively. In this study, acid phosphatase and urease enzyme activities increased in the inoculated treatments compared to the control.
Conclusions
The AM fungal diversity in the plant rhizospheres was variable among the different plant species in fly ash pond. G. rosea was the dominant fungus from these soils. Among the six plant species, L. camara was associated to all identified AM fungal species except G. magnicaule compared to other plants. The occurrence of AM fungi in low numbers in rhizosphere soil and in roots of plants growing in fly ash is an indication of their adaptation to fly ash. Inoculation of AM fungi improved growth and nutrient uptake of E. tereticornis seedlings and reduced translocation of metals into shoot portion. The application of fly ash adapted AM fungal species may aid in the environmental management and greening of fly ash ponds.
References
Appoloni S, Lekberg Y, Tercek MT, Zabinski CA, Redecker D (2008) Molecular community analysis of arbuscular mycorrhiza fungi in roots of geothermal soils in Yellowstone National Park (USA). Microb Ecol 56:649–659
Arnon DE (1949) Copper enzyme in isolated chloroplast, polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1
Arriagadaa CA, Herrerab MA, Ocampo JA (2007) Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. J Environ Manag 84:93–99
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Bedini S, Turrini A, Rigo C, Argese E, Giovannetti M (2010) Molecular characterization and glomalin production of arbuscular mycorrhizal fungi colonizing a heavy metal polluted ash disposal island, downtown Venice. Soil Biol Biochem 42:758–765
Bray RH, Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Chen BD, Christie P, Li XL (2001) A modified glass bead compartment cultivation system for studies on nutrient uptake by arbuscular mycorrhiza. Chemosphere 42:185–192
Clapp JP, Young JPW, Merryweather JW, Fitter AH (1995) Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. New Phytol 130:259–265
Cumming JR, Ning J (2003) Arbuscular mycorrhizal fungi enhance aluminium resistance of broomsedge (Andropogon virginicus L.). J Exp Bot 54:1447–1459
Enkhtuya B, Rydlová J, Vosátka M (2000) Effectiveness of indigenous and non-indigenous isolates of arbuscular mycorrhizal fungi in soils from degraded ecosystems and man-made habitats. Appl Soil Ecol 14:201–211
Enkhtuya B, Poschl M, Vosatka M (2005) Native grass facilitates mycorrhizal colonization and P uptake of tree seedlings in two anthropogenic substrates. Water Air Soil Pollut 166:217–236
Eom AH, David C, Hartnett A, Gail WT, Wilson C (2000) Host plant species effects on arbuscular mycorrhizal fungal communities in tall grass prairie. Oecologia 122:435–444
Galván GA, Parádi I, Burger K, Baar J, Kuyper TW, Scholten OE, Kik C (2009) Molecular diversity of arbuscular mycorrhizal fungi in onion roots from organic and conventional farming systems in the Netherlands. Mycorrhiza 19:317–328
Gerdemann JW, Nicholson TH (1963) Spores of mycorrhizal endogone species extracted from the soil by wet sieving and decanting. Trans Br Mycol Soc 46:2345–2440
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
González-Chávez MC, Carrillo-González R, Wright SF, Nichols KA (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323
Guadarrama P, Alvarez-Sanchez FJ (1999) Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza 8:267–270
Haynes RJ (2009) Reclamation and revegetation of fly ash disposal sites: challenges and research needs. J Environ Manag 90:43–53
Juwarkar AA, Jambhulkar HP (2008) Restoration of fly ash dump through biological interventions. Environ Monit Assess 139:355–365
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298
Li T, Li L, Sha T, Zhang H, Zhao Z (2010) Molecular diversity of arbuscular mycorrhizal fungi associated with two dominant xerophytes in a valley-type savanna, southwest China. Appl Soil Ecol 44:61–66
Lorgio EA, Julio RG, Peter LM (1999) Variation in soil microorganisms and nutrients underneath and outside the canopy of Adesimia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average rainfall. J Arid Environ 42:61–70
McGarity JW, Myers MG (1967) A survey of urease activity in soils of northern New South Wales. Plant Soil 27:217–238
Ministry of Environment and Forests (MOEF) Notification (2007) Fly Ash Notification 2007. Ministry of Environment and Forests, New Delhi
Morton JB (1993) Problems and solution for the integration of glomalean taxonomy, systematic biology, and the study of endomycorrhizal phenomena. Mycorrhiza 2:97–109
Mosse B (1975) Specificity in VA mycorrhizas. In: Sander FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic Press, London, pp 469–484
Ning J (2000) Mycorrhizal roles in broomsedge plants under phosphorus limitation and aluminum toxicity. PhD dissertation, West Virginia University, Morgantown, WV
Oliveira RS, Boyer LB, Carvalho MF, Jeffries P, Vosátka M, Castro PML, Dodd JC (2010) Genetic, phenotypic and functional variation within a Glomus geosporum isolate cultivated with or without the stress of a highly alkaline anthropogenic sediment. Appl Soil Ecol 45:39–48
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Ouzounidou G (1995) Cu-ions mediated changes in growth, chlorophyll and other ion contents in a Cu-tolerant Koeleria splendens. Biol Plantarum 37:71–78
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis, Part 2D. Chemical and microbiological properties, 2nd edn. Agronomy No. 9, ASA. SSSA, Madison
Pennisi E (2008) Proposal to ‘wikify’ GeeBank meets stiff resistance. Science 319:1598–1599
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Pringle A, Bever AJ (2002) Divergent phenologies may facilitate the coexistence of arbuscular mycorrhizal fungi in a North Carolina grassland. Am J Bot 89:1439–1446
Redecker D (2000) Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10:73–80
Redecker D, Hijri I, Wiemken A (2003) Molecular identification of arbuscular mycorrhizal fungi in roots: perspectives and problems. Folia Geobot 38:113–124
Renker C, Blanke V, Buscot F (2005) Diversity of arbuscular mycorrhizal fungi in grassland spontaneously developed on area polluted by a fertilizer plant. Environ Pollut 135:255–266
Rezaee Danesh Y, Mohammadi Goltapeh IE, Alizadeh A, Varma A, Mukerjii KG (2007) Arbuscular-mycorrhizal fungi associated with alfalfa rhizosphere in Iran. Am-Eur J Agric Environ Sci 2:574–580
Robinson-Boyer L, Grzyb I, Jeffries P (2009) Shifting the balance from qualitative to quantitative analysis of arbuscular mycorrhizal communities in field soils. Fungal Ecol 2:1–9
Schenck NC, Pérez Y (1990) Manual for identification of vesicular arbuscular mycorrhizal fungi. INVAM, University of Florida, Gainesville
Selvam A, Mahadevan A (2002) Distribution of mycorrhizas in an abandoned fly ash pond and mined sites of Neyveli Lignite Corporation, Tamil Nadu, India. Basic Appl Ecol 3:277–284
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, Cambridge
Sylvia DM, Williams SE (1992) Vesicular-arbuscular mycorrhizae and environmental stresses. In: Bethlenfalvay GJ, Linderman RG (eds) Mycorrhizae in sustainable agriculture. ASA, Madison, WI, pp 101–124
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Vivas A, Barea JM, Azcón R (2005) Interactive effect of Brevibacillus brevis and Glomus mosseae, both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soil. Environ Pollut 134:257–266
Vosatka M (2001) A future role for the use of arbuscular mycorrhizal fungi in soil remediation: a chance for small-medium enterprises? Minerva Biotechnol 13:69–72
Walker C (1992) Systematics and taxonomy of the arbuscular endomycorrhizal fungi (Glomales)—a possible way forward. Agronomie 12:887–897
Walkley AJ, Black IA (1934) Estimation of soil organic carbon by the chromic acid titration method. Soil Sci 37:29–38
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, San Diego, CA, pp 315–322
Wong MH, Wong JWC (1990) Effects of fly-ash on yields and elemental composition of two vegetables, Brassica parachinensis and B. chinensis. Agric Ecosys Environ 30:25
Wu FY, Bi YL, Wong MH (2009) Dual inoculation with an arbuscular mycorrhizal fungus and rhizobium to facilitate the growth of Alfalfa on coal mine substrates. J Plant Nutr 32:755–771
Zarei M, Hempel S, Wubet T, Schäfer T, Savaghebi Gh, Jouzani GhS, Nekouei MK, Buscot F (2010) Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ Pollut 158:2757–2765
Acknowledgments
The authors are grateful to TIFAC-CORE, Thapar University and NALCO, Damanjodi, Orissa, India, for facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giridhar Babu, A., Sudhakara Reddy, M. Diversity of Arbuscular Mycorrhizal Fungi Associated with Plants Growing in Fly Ash Pond and Their Potential Role in Ecological Restoration. Curr Microbiol 63, 273–280 (2011). https://doi.org/10.1007/s00284-011-9974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-011-9974-5