Abstract
Despite discovery of the pathogen more than 100 years ago, tuberculosis (TB) continues to be a major killer disease worldwide. Currently a third of world population is infected and multiple-drug-resistant (mdr) TB registers maximum mortality by a single pathogen. Nanomedicine provides enormous opportunity for developing novel drugs. We have recently demonstrated surface-modified-lipophilic-nanosilica as drug to combat malaria and 100% lethal virus, BmNPV. Nanosilver possesses inherent antibacterial properties, but toxicity is a major concern. We hypothesized that capping with nature-inspired biomolecules, bovine serum albumin (BSA) and Poly-n-vinyl-pyrrolidone (PVP) used as blood volume extender, might insure biosafety. BSA-nano-Ag was found to be more stable than PVP-nano-Ag at physiological pH. In this first ever study on clinical isolates collected from TB endemic areas, we report, BSA-nano-Ag act as potent anti-TB drug. Further study with (human serum albumin)-nano-Ag and core–shell-nano-Ag could increase the biocompatibility of oral TB drug formulations without compromising on the efficacy of the drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomedicine provides enormous opportunity for developing novel drugs. We have recently demonstrated that surface-modified-lipophilic-nanosilica could be effectively used to combat malaria [1–5] and 100% lethal virus, BmNPV [5, 6]. We have also reported novel mechanisms of action of these nanoparticles [7].
Adverse drug reactions (ADR) of commonly used anti-TB drugs, worldwide like Streptomycin, Rifampicin, Pyrazinamide, and INH ranges from intolerance to hepatitis, neuropathy, optic nerve atrophy, etc. They require regular monitoring like liver function tests. Search for safer drugs propelled this study. Nanoscience derived tools like surface capping with natural biomolecule like Bovine Serum Albumin (BSA) and biocompatible chemical like Poly Vinyl Pyrrolidine (PVP) were considered to be valid options. It was anticipated that saturated surface capping would be able to mask the pure nano-Ag surface and thus would reduce toxicity without hampering efficacy. Before, the efficacy of the putative oral drugs is explored, stability at different pH is considered to be the most important parameter for oral administration as the molecule would experience large variation in pH starting from esophagus to large intestine and other target tissues in the body. Moreover, body uptake of drugs is also dependent upon pH. This study, therefore, first observed the stability of the BSA- and PVP-capped nano-Ag at different pH and then explored the potential of these putative drugs toward growth inhibition of control TB strains (Mycobacterium tuberculosis) as well as strains isolated from clinical specimens like sputum or body fluids from one of the largest TB endemic epicenters of India. The effectiveness of BSA-capped nano-Ag and its mechanism of action against the mdr strains of M. tuberculosis (identified on the basis of conventional drug sensitivity testing) has been included in this study. Encouragingly, the results indicate that this novel design could be further extended for developing improved medicinal agents for combating multi-drug-resistant TB.
Materials and Methods
All experimental studies with TB unless otherwise mentioned were outsourced and performed at the biosafety facilities in the Pathowynd Clinical Laboratory Inc., Kolkata, India, which follows current laws and protocols for biosafety in India.
Preparation of Aqueous Solution of Nano-Ag
Nano-Ag directly conjugated to natural BSA protein molecules were produced as per standard protocol [8]. Silver nitrate (AgNO3), sodium borohydride (NaBH4, 95%), and 200 proof spectrophotometric-grade ethanol were purchased from Fisher (99.9%, HPLC grade). Bovine serum albumin (BSA, M.W 66,000) was purchased from Calbiochem and used without further treatment. Briefly, sodium borohydride (0.0023 g in 1 ml of water) was added to an aqueous solution of silver nitrate (0.0162 g) and BSA (0.0356 g) under vigorous stirring of 1000 rpm by a magnetic stirrer. The molar ratio of Ag+: BSA was 28:1, and the molar ratio of Ag+:BH4− was 1:1. The reaction volume was 40 ml, and contained 13.50 μmol BSA. The reaction was allowed to proceed for 1 h, and the product was purified by precipitation at −5°C, followed by cold ethanol filtration. Nanopure water (18.2 m≏) from Sartorius was used throughout the experiment. Poly-n-vinyl-pyrrolidone (PVP, Mw = 40,000) purchased form Aldrich was used to prepare PVP-capped nano-Ag. The silver sol was prepared by reduction of Ag + ions in presence of ethanolic solution of PVP. The weight ratios of metal salt to PVP were 1:5, 1:10, and 1:20. The solution was kept under vigorous stirring and a clear yellow solution was obtained. The reduction was carried out at 70°C in a thermostatic water bath with reflux unit for 30 min [9].
Characterization of BSA- and PVP-Capped Nano-Ag
BSA- and PVP-capped nano-Ag particles were subjected to UV–VIS, DLS, TEM, and SEM analyses. 60 μl of silver nanoparticles was mixed in 3 ml of nanopure water (Arium 61316 and 611VF, Sartorius Inc., USA). UV–Visible absorption spectra (Perkin-Elmer Inc., USA; model: double beam lambda 25) were recorded with a 1-cm quartz cell. DLS (Malvern instruments, UK; model nano-S) analysis was done to study the size distribution of BSA- and PVP-capped nano-Ag. Further characterization was done by Transmission Electron Microscopy (TEM) (Joel Inc., USA; model JEM 2010). For transmission electron microscopy (TEM) studies, 15 μl of BSA- and PVP-capped nano-Ag particles were deposited on copper grids (Pro Sci Tech Inc., Australia; 300 mesh) under laminar hood and were allowed to air dry in a UV-sterilized condition and data were collected. During scanning electron microscopy (SEM) studies, Mycobacterium smears were prepared on 1 × 1 cm xylene cleaned glass slides and air dried followed by overnight glutaraldehyde fixation at 4°C. Each slide was coated with gold–palladium at 5 mA by SC7620 sputter-coater. The samples were analyzed by SEM-FEI QUANTA 200 (FEI Inc., USA). Stability of BSA- and PVP-capped nano-Ag in physiologically relevant salt solution (0.9% pure NaCl), temperature (37–80°C), and serum (Bovine serum, GE Healthcare) was monitored by UV–VIS for 1 h and time series data were generated. Similarly, structural stability of BSA- and PVP-capped nano-Ag with change in pH (fine probe pH meter; Sartorius; model PB 11) and temperature over 24 h time were recorded. Structural stability of BSA- and PVP-capped nano-Ag in salt, serum, different pH and temperature was studied by UV–VIS and DLS, as described above.
Clinical TB Samples
Sputum samples were collected from the pulmonary TB patients visiting state hospital outdoor clinics. Body fluids (pleural and peritoneal) are collected from extra-pulmonary TB patients visiting pathology department of State hospitals. All samples were collected maintaining proper sterility. Bioethical permissions from respective departments were obtained before collection of samples. Control strains like H37RVof M. tuberculosis and M. xenopi were obtained from the National Culture Collection of Tuberculosis Research Centre, India. Sputum samples were liquefied and decontaminated using equal volume of a mixture of 4% NaOH and N-acetyl-L-cysteine (NALC) for 30 min at room temperature. After decontamination, sample was neutralized by sterile PBS (pH 6.8) and centrifuged at 3600 g for 30 min. Supernatant was discarded and loose pellet was resuspended in sterile distilled water [10]. The body fluids were concentrated by spinning down the whole fluid, discarding the supernatant and collecting the pellet maintaining sterility for further use [11]. Resuspended pellets of all specimens (sputum and body fluids) were stained by standard Ziehl-Neelson (Z-N) method for demonstration of Acid Fast Bacilli (AFB) [AFB Kit, Himedia Lab, India] and were examined for the presence of AFB with microscope (oil-immersion; Zeiss Axiolab, Germany). Smears recorded as positive in standard AFB scale and data per 100 fields were collected as per RNTCP (Revised National Tuberculosis Control Program, WHO) guidelines as described in Table 1 [12].
In Vitro Culture of Mycobacterium tuberculosis
Sterile clinical specimens, standard strains of H37 RV of M. tuberculosis and M. xenopi were grown both in Lowenstein-Jensen (L-J) slants (solid media) and Middlebrook 7H9 broth (liquid media) incubated for 5 weeks (solid media) and 3 weeks (liquid media) (Fig. 1). Cultures were grown at 35°C in dark within an atmosphere of 5% CO2 and 70% humidity (RH). Standard colony and surface pellicle counting was done [13]. Figure 1a shows the typical Tb collection vials from the clinical specimens.
a Mycobacterium culture, b Niacin test, c Nitrate test, d Catalase test. e Multiplex PCR amplification of 165, 365, and 451bp regions if the clinical specimens and control strains of M. tuberculosis and Non tubular mycobacteria (MOTT) on 2% agarose gel. Lane 1 pGEM molecular marker, lane 2–3 patients' sample (Pleural fluid), lane 4 H37RV (control strain) of M. tuberculosis, lane 5–6 patients' sample (Peritonial fluid), lane 7–9 other Atypical mycobacteria, lane 10–12 sputum sample of patients, lane 13 positive control, lane 14 negative control. f Multiplex PCR amplification of 165, 365, and 451 bp regions if the clinical specimens of M. tuberculosis and Non tubercular mycobacterium on 2.5% agarose gel. Lane 1 100 bp molecular marker, lane 2–12 patient samples, lane 13 positive control, lane 14 negative control
Biochemical Assays and PCR
Conventional biochemical assays, viz., nitrate reduction, catalase production, and niacin production were done for identification of different strains of M. tuberculosis as per kit protocols supplied by manufacturer (Himedia Lab, India) (Fig. 1) [14]. Genomic DNA was mini-prepped from aforesaid pelleted clinical specimens as well as control strains (colony and broth) using modified lysis method [13]. Multiplex PCR using three pairs of oligonucleotide primers representing 65 kd Hsp protein, Dnaj gene, and IS6110 with expected band sizes of 165, 365, and 541 bp, respectively, were performed (Himedia Lab, India) [15]. Control M. tuberculosis are expected to show amplification of all three targets, whereas atypical Mycobacteria like M. xenopi (MOTT organisms) would show amplification of one or two target sequences. Figure 1e, f shows results of multiplex PCR used for typing all TB samples.
Antimicrobial Properties of Nano-Silver
Three TB clinical specimens isolated from three different sites, i.e., from sputum, peritoneal, and pleural fluid, respectively, and two control strains of M. tuberculosis and M. xenopi were included in the study. Minimum inhibitory concentration (MIC) of BSA- and PVP-capped nano-Ag particles against clinical and control samples were determined. All strains were inoculated in two batches of 5 ml Middlebrook 7H9 broth. 1.6, 4, 8 μg/ml of nanoparticles were added immediately to the first batch. In the second batch, same graded concentrations of nanoparticles (1.6, 4, and 8 μg/ml) were added to the 20 days old broth culture to demonstrate the subsequent growth inhibition or lysis of bacteria by nanoparticles and were monitored for another 4 weeks. Bacterial growth was confirmed by appearance of turbidity in the media Z-N staining from centrifuged deposits and subsequent appearance of colonies on subculture on L-J slant. As already mentioned in order to quantify the amount of growth, RNTCP (Revised National Tuberculosis Control Program) guidelines were used for AFB smears [12]. Positive control experiments utilized bacterial inoculums without any nanoparticles and negative control contained only nanoparticles (8 μg/ml) without any bacterial inoculums.
Microarray Experiments
All microarray experiments were outsourced to Agilent Technologies Inc. and done in triplicate on Agilent platforms. Following nanoparticles were used for this experiment—BSA-capped nano-Ag, nano-silica, nano-aluminosilicate, nano-ZnO, nano-gold. Except BSA-capped nano-Ag, similar sized were custom made from M/S MKNANO Inc, Canada. One-color protocols were used where one labeled RNA sample was hybridized to each microarray. Agilent used its own method or software to generate a quantitative signal value and a qualitative detection call for each probe on the microarray. Csdg wild-type strain of Drosophila melanogaster were fed with nanoparticles 8 μg/ml with standard maize meal (added after autoclaving in sterile condition) for a period of 15 days, and RNA were prepared by Agilent Technologies. Before and after hybridization experiments, all quality control parameters were tested in triplicate by Agilent Technologies following the manufacturer’s protocols.
Results and Discussion
Despite the discovery of the M. tuberculosis more than a hundred years ago, tuberculosis (TB) continues to be a major killer disease worldwide. Currently about a third of the world population is infected with TB and related pathogens. It registers the highest mortality by a single pathogen [16] accounting for about 3 million deaths and about 10 million new cases each year. This corresponds to more than 7000 deaths per day and 1000 new cases per hour each day [17]. In India each year, about 2 million patients develop active disease and up to half a million die [18]. Moreover, emergence of multi-drug-resistant (mdr) strains and their sinister association with HIV/AIDS has posed a serious threat to the TB control program worldwide [19]. mdr is associated with high mortality rate of 50–80% and spans a relatively short time (4–16 weeks) from diagnosis to death [20]. Chemotherapy for TB was possible with the discovery of streptomycin in mid-1940s. The introduction of Rifampicin in the early 1970s heralded the era of effective short course treatment. Pyrazinamide and INH also contributed much to it. But, even today effective chemotherapy requires 6 to 18 months of treatment depending on the nature and site of the disease. Failure of remission by first line of drugs in 3–4 months requires introduction of a second line of drugs. Anti-tubercular drugs have serious Adverse Drug Reactions (ADR) including intolerance, hepatitis, neuropathy, optic nerve atrophy, etc. They should be used with extreme caution in pregnancy and lactation due to narrow margin of safety. Moreover, continuous monitoring of liver function test, neurological deficiencies, etc. is required. The total cost of the treatment is also high considering the relatively long span of treatment [21].
The anti-microbial property of colloidal silver is well known since long and a considerable amount of investigations has been carried out to establish its potential application in therapeutics [22]. But, little information is available as of date regarding its stability in different biological fluids, physiological salt solution, pH, and thermal stability. Colloidal silver is toxic and we hypothesized that surface capping with natural ligands like BSA might also reduce the toxicity. While considering nanosilver as a putative drug and controlled release thereof [23, 24], knowledge of stability is of paramount importance. Moreover, research efforts on the efficacy of the nanosilver on clinical specimens are scanty.
Characterization of BSA- and PVP-Capped Nano-Ag
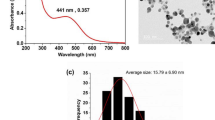
It was previously demonstrated that a wide range of silver nanoparticles could be produced by varying the ratio of Ag and BSA/PVP. The size range of BSA-capped silver nanoparticle (BSA-Ag) was found to be 5–9 nm. On measuring TEM images of 100 particles, the average size was estimated to be 5.74 nm (Fig. 2).The inset picture in Fig 2 shows the characteristic diffraction pattern of BSA-capped nano-Ag. BSA-capped nano-Ag shows surface plasmon peak at 401.1 nm. BSA-capped nano-Ag particles were found to be stable at physiological salt solution like L-J media and Middle-brooks media, with a minor shift of the UV–VIS peak by 3 nm (Fig. 3). Stability studies were monitored for 24 h.
Silver nanoparticles were directly conjugated to BSA protein molecules in aqueous solution. Serum albumin is a globular protein, and is the most-abundant protein in blood plasma. Bovine serum albumin (BSA) is a single polypeptide chain composed of 583 amino acid residues. The strongest interactions of BSA with silver would likely involve 35 thiol-bearing cysteine residues [8]. This is probably due to the fact that Ag and S being highly polarisable atoms, a very stable soft acid (Ag)–soft base (S) interaction takes place in accordance with the HSAB (Hard Soft Acid Base) principle. It is noteworthy that Ag has been found to form stable compounds like AgNO3, Ag2S, etc. Using sodium borohydride, a strong reducing agent, BSA was stabilized on nano-Ag via direct bonding with these thiol bearing cysteine residues and this might have provided steric protection due to the bulkiness of the protein.
PVP is a linear polymer and stabilizes the nanoparticle surface via bonding with the pyrrolidone ring. Infrared (IR) and X-ray photoelectron spectroscopy (XPS) studies have revealed that both oxygen and nitrogen atoms of the pyrrolidone ring can promote the adsorption of PVP chains onto the surface of silver (data not shown).
PVP-capped silver nanoparticles (PVP nano-Ag) show a size distribution of 6–45 nm with an average size of 28.33 nm (Fig. 4). PVP-capped nano-Ag shows typical surface plasmon peak at 423 nm. PVP-capped nano-Ag begins to destabilize pH 7.0 onward in L-J media and Middle-brooks media (Fig. 5). PVP-capped nano-Ag were found to be more stable at elevated temperatures (>60°C) (data not shown). BSA-capped and PVP-capped nano-Ag particles were found to be equally stable in the physiological body temperature range. Salt (0.9% pure NaCl) and serum (20% solution of commercially available serum) did not induce aggregation of both BSA- and PVP-capped nano-Ag particles (Fig. 6).
Therefore, based on the aforesaid stability studies BSA-capped nano-Ag were used for further anti-mycobacterium efficacy studies in vitro (data not shown) and in vivo.
Inhibitory Assay of BSA-Ag on M. tuberculosis
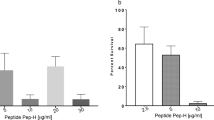
Five different strains of Mycobacterium have been used in this study, of which four are of M. tuberculosis (one is a control strain, i.e., H37RV and three others are clinical isolates from sputum and body fluids of TB patients). The fifth strain is of M. xenopi, which is an atypical or MOTT (Mycobacterium other than TB) organism causing opportunistic infection in immunocompromised hosts [21]. All experiments were done with at least 5–6 replications unless otherwise specifically mentioned. In the first set of each batch, the bacterial inoculums were added in three tubes containing Middlebrook’s broth and increasing concentration of nanosilver particles (8, 20, and 40 μg in 5 ml broth). A positive control set by a pure broth is culture of a Mycobacterial strain without any nanoparticle, whereas the negative control contained only 100 μg of nanosilver particles without any inoculums. In the second set, 1.6, 4, 8 μg/ml broth, BSA-capped nano-Ag were added in 20 days old broth culture (judged by O. D. value at 650 nm, appearance of turbidity, AFB tests) and lytic effect of the nanosilver particles were studied 20 day old established cultures (Table 2). M. xenopi shows complete growth inhibition in all the concentrations of nanosilver. No strain of M. tuberculosis showed any growth inhibition at concentrations of 1.6 μg/ml (8 μg/ml). At 4 μg/ml concentration, growth is partially inhibited in different strains of M. tuberculosis whereas in (8 μg/ml) complete growth inhibition occurred for all four strains (Table 3). In the next batch, effect of nanosilver particles is observed after 20 days of addition of nanoparticles. At 1.6 μg/ml concentration, there was almost no lysis except in M. xenopi (showing a scanty growth even in the positive control) At 4 μg/ml concentration, lysis was observed in the first two tubes (isolates from clinical specimens) but not in the culture containing isolates from the pleural fluid and the control strain. At the highest concentration, complete lysis was observed in all plates (Table 3). A comparative study of growth inhibition by BSA-capped nano-silver particles along with standard anti tubercular drugs (Rifampicin and INH) are compiled in Table 4. The strain isolated from the pleural fluid showed in vivo resistance to both Rifampicin (2 μg/ml) and INH (5 μg/ml) and by definition of pharmacology, it can be marked as a Multi Drug Resistant (mdr) strain. But even this strain is sensitive to 8 μg/ml concentration of BSA-capped nano-Ag.
In order to decipher the mechanism of action of silver nanoparticles, UV–VIS, SEM, and TEM studies were performed. BSA-capped nano-Ag alone shows peak at 401.1 nm and Mycobacterium alone gives a peak at 580 nm. We hypothesize that Mycobacterium and control BSA-capped nano-Ag particles conjugation complex gives the intermediate peak at 421 nm (Fig. 7). High resolution (HR) SEM studies revealed that BSA-capped nano-Ag wrinkles the cell surface and affects the integrity of the surface of the bacterial cell wall (Fig. 8). Eventually, the cytoplasmic material is extruded from the cell leading to the collapse of the cell. The collapsed cell then coalesces to form an amorphous mass of cell debris (data not shown). Figure 9a shows the high resolution TEM images of the surface of control Mycobacterium and Fig. 9b shows the data on the interaction of the BSA-capped nano-Ag and the bacteria. The inset in Fig. 9a shows turbidity clearly indicating the growth of TB bacteria whereas the inset in Fig. 9b is control. Further, low and HR-TEM studies revealed that BSA-capped nano-Ag particles enter inside the Mycobacterium cell (Fig. 10a , b) and were found to form precipitates as indicated by arrows (Fig. 10c, d). Therefore, BSA-capped nano-Ag kills Mycobacterium in a completely different fashion from that of antibiotics commonly used for treating TB. We may conclude that these nanaoparticles damage the cell wall and also on gaining entry into the cell tend to disrupt physiological processes via pathways ill understood at present. The combination of both these effects might be the reason for the startling observation that mdr strains fail to survive at higher dose of nanosilver. At present, we are investigating the cellular mechanisms.
In order to assess biosafety, we have conducted microarray studies in the fruit fly model. The effects of nano-silica, nano-alumino-silicate, nano-gold, nano-ZnO vis a vis BSA-capped nano-Ag were compared at the genome wide level. Here, we show the effects of the nanoparticles on 63 taste/gustatory (Gr) receptors of Drosophila melanogaster (Table 5). Till now, the ligands of a handful of Gr-s are known. Results indicate that the effects of BSA-capped nano-Ag as a drug on the fly genome is random and therefore could be considered safe. Long-term animal studies are in progress.
To the best of our knowledge, this is the first successful clinical demonstration of fighting M. tuberculosis including mdr strains with the help of surface-modified nanosilver. We hope this approach might usher a novel strategy for fighting lethal TB in the near future. Further research on the development of different intelligently surface-modified nanosilver as well as core–shell nano-silver which might reduce bio-toxicity to a very low level is currently underway. It has not escaped our attention that use of human serum albumin would be a better choice while developing drug formulations later.
References
Goswami A, Singh S, Redkar VD, Sharma S (1997) Characterization of P0, a ribosomal phosphoprotein of Plasmodium falciparum antibody against amino-terminal domain inhibits parasite growth. J Biol Chem 272(18):12138–12143
Sharma S, Goswami A, Singh NJ, Kabilan L, Deodhar SS (1996) Immunogenicity of the nonrepetitive regions of the circumsporozoite protein of Plasmodium knowlesi. Am J Trop Med Hyg 55(6):635–641
Goswami A, Chatterjee S, Sharma S (1996) Cloning of a ribosomal phosphoprotein P0 gene homologue from Plasmodium falciparum. Mol Biochem Parasitol (Elsevier) 82(1):117–120
Singh S, Sehgal A, Goswami A, Waghmare S, Chakrabarty T, Sharma S (2002) Surface expression of the conserved ribosomal protein P0 on parasite and other cells. Mol Biochem Parasitol (Elsevier) 119(1):121–124
Rahman A, Seth D, Mukhopadhyaya SK, Brahmachary RL, Ulrichs C, Goswami A (2009) Surface functionalized amorphous nanosilica and microsilica with nanopores as promising tools in biomedicine. Naturwissenschaften 96:31–38
Goswami A, Rahman A, Biswas N, Ulrichs C, Büttner C, Bramhachary RL, Datta A (2009) Nanoparticle–virus complex shows enhanced immunological effect against baculovirus. J Nanosci Nanotechnol 9:1–5
Ulrichs C, Welke B, Mucha-Pelzer T, Goswami A, Mewis I (2008) Effect of solid particulate matter deposits on vegetation: a review. Function Plant Sci Biotechnol 2(1):56–62
Elechiguerra JL, Burt JL, Morones JR, Bragado AC, Gao X, Lara HH, Yacaman MJ (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 3:6
Kim JS (2007) Reduction of Silver Nitrate in Ethanol by Poly(N-vinylpyrrolidone). J Ind Eng Chem 13(4):566–570
Kubica GP, Dye WE, Cohn ML, Middlebrook G (1963) Sputum digestion and decontamination with N-acetyl L- cysteine sodium hydroxide for culture of bacteria. Am Rev Respir Dis 87:775–779
Forbes BA, Sahm DF, Weissfeld AS (eds) (2004) Baily & Scott’s diagnostic microbiology, 2nd edn. Mosby Elsevier, St louis, MO, pp 488–506
World Health Organisation (2003) Treatment of tuberculosis: guidelines for national programmes, 3rd edn. World Health Organisation Geneva, Switzerland
Wan B, Rayner A, Harris G (2006) Mycobacterium. In: College JG, Fraser AG, Marmion BP, Simmons A (eds) Mackie & Mc Cartney’s practical medical microbiology, 14th edn. Churchill Livingstone, London, UK, pp 332–335
Sritharan V, Barker RH (1991) A simple method for diagnosis of M. tuberculosis infection in clinical samples using PCR. Mol Cell Probes 5:585–595
Bandopadhyay D, Gupta S, Banerjee S, Ray D, Bhattacharya S, Bhattacharya B (2008) Adenosine deaminase estimation and multiplex polymerase chain reaction in diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis 12(10):1203–1208
World Health Organization. Global Tuberculosis Control, Surveillance, Planning, Financing. WHO/CDS/Tuberculosis/2002.295
World Health Organization. Global Tuberculosis Control, Surveillance, Planning, Financing. WHO/HTM/Tuberculosis/2005.349.Geneva, Switzerland: WHO, 2005
World Health Organization. Prevalence and incidence of Tuberculosis in India: A comprehensive Review, 1997. World Health Organization. Global Tuberculosis Control, Surveillance, Planning, Financing. WHO/CDS/Tuberculosis/2002.295,1998
Barnes P, Blotch AB, Davidson BT, Snyder DE Jr (1991) Tuberculosis in patients with immunodeficiency virus infection. N Eng J Med 324:1644–1650
Dooley SW, Jarvis WR, Martone WJ, Snyder DE (1992) Multidrug resistant tuberculosis (editorial). Ann Intern Med 117:191–196
Raviglione MC, O’Brien RJ (1998) Tuberculosis. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL (eds) Harrison’s principles of internal medicine, 14th edn. Mc Graw Hill Companies, Inc, Columbus, USA, pp 1010–1016
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176(1):1–12
Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Baystonl R, Brown PD, Winship PD, Reid HJ (2004) Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? Nanomedicine: Nanotechnol Biol Med 3(1):95–101
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:9–16 225103
Acknowledgments
This study was supported by Indian Statistical Institute (ISI) intramural grant on nanoscience for 2008-2011, NAIP-ICAR-World Bank mega-project grant no. NAIP/Comp-4/C3004/2008-2009 (PI: A. G.). D. S. was supported by DBT nanoscience infrastructure mega grant no. BT/PR9050/NNT/28/21/2007 (PI: A. G.) and DBT exploratory grant on Nanocomposites no. BT/PR8931/NNT/28/07/2007(PI,ISI:A.G.). This article has been critically examined by Mrs. Indrani Roy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seth, D., Choudhury, S.R., Pradhan, S. et al. Nature-inspired Novel Drug Design Paradigm Using Nanosilver: Efficacy on Multi-Drug-Resistant Clinical Isolates of Tuberculosis. Curr Microbiol 62, 715–726 (2011). https://doi.org/10.1007/s00284-010-9770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9770-7