Abstract
Salmonella enterica serovar Typhi z66 positive strain contains a fljBA-like operon on a linear plasmid. The operon contains the gene fljB:z66 which encodes the z66 antigen. RpoE is a sigma factor σE that initiates transcription of a series of genes in Escherichia and Salmonella under environmental stresses. To investigate whether the gene fljB:z66 is regulated by RpoE (σE), a rpoE deletion mutant of S. enterica serovar Typhi (ΔrpoE) was prepared in this study. The defective motility of the ΔrpoE was confirmed firstly. Transcriptional expression of flagellar genes was screened using a genomic DNA microarray. Some class-2 and most class-3 flagellar genes were downregulated in the ΔrpoE after 30 min of hyperosmotic stress. The expression of fliA and fljB:z66, a class-2 flagellar gene and a class-3 flagellar gene, obviously decreased; however, expression of the class-1 flagellar genes flhDC did not change obviously in the ΔrpoE compared to the wild-type strain in the same conditions. Results of quantitative real-time PCR (qRT-PCR) showed that the expression levels of fliA and fljB:z66 in the ΔrpoE after 30 min of hyperosmotic stress decreased about five and eightfold, respectively, compared to the wild-type strain. Similar results were observed at 120 min of hyperosmotic stress. Western blotting and qRT-PCR analysis showed that expression of fliA and fljB:z66 was significantly increased after supplemental expression of rpoE with a recombinant plasmid pBADrpoE in the ΔrpoE strain. These results demonstrated that RpoE promoted the expression of class-3 flagellar genes and it might be performed by initiating the expression of fliA in S. enterica serovar Typhi under hyperosmotic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flagellum is an important pathogenic factor that is essential for the motility of Salmonella. Approximately 50 genes are associated with flagellar structure and function, and they are divided into three classes according to the regulation of transcription. The flhDC operon lies at the top of the hierarchy as the sole class-1 operon, with both of its gene products being absolutely required for the expression of all other genes in the flagellar regulon, e.g., those belonging to the class-2 and class-3 operons. FliA is required for expression of class-3 operons such as fljBA [13, 14]. Most Salmonella enterica serovars have two flagellin genes, fliC and fljB, that are alternately expressed producing phase variation. S. enterica serovar Typhi (S. Typhi), the pathogen responsible for typhoid fever in humans, was once considered a monophasic strain because it only carries fliC [7]. However, the z66 antigen-positive strain of S. Typhi has a special fljBA gene cluster located on a novel 27-kb linear plasmid [5]. The fljB has been identified as the gene encoding the z66 antigen, and fljA has been verified as the fliC repressor gene [4, 10, 30].
RpoE, a sigma factor σE encoded by the gene rpoE, is an extracytoplasmic factor that initiates transcription of a series of genes in Escherichia and Salmonella under environmental stresses, e.g., high osmolarity, oxidative stress [18, 21, 25]. RpoE is required to maintain cell envelope integrity in Escherichia coli [9]. In unstressed conditions, RpoE is repressed by RseA, an anti-sigma factor which is located on the inner membrane of the bacterial cell and can bind to RpoE [8]. In some stress conditions, RseA can be degraded by inner membrane proteases RseP and DegS, which are activated by unfolded outer membrane proteins (OMPs) [2, 3]. Sequential degradation of RseA releases RpoE from the cell inner membrane. As expected from its role in the stress response, the RpoE regulon includes genes encoding periplasmic foldases, proteases, and chaperones that assist in OMP folding. Much information is available on the role of RpoE in the response to cell envelope stress and on the identification of the RpoE regulon [1, 20]. However, reports about the regulation of RpoE on flagella are scarce.
In a previous genomic microarray analysis of gene expression in S. Typhi under high osmotic stress, we found that the expression of rpoE decreased at the early stage of stress and was restored at a later stage. The expression kinetics of fljB:z66 and many flagellar genes were similar to that of rpoE [11]. This elicited the proposal that RpoE might be associated with regulation of the expression of fljB:z66 and other flagellar genes in S. Typhi during osmotic stress. To verify the proposal, in this study, genomic DNA microarray was utilized to explore the expression of flagellar genes in the mutant after 30 min of hyperosmotic stress. We found that fliA and most class-3 flagellar genes were downregulated, but flhDC and some class-2 flagellar genes were unchanged in the rpoE deletion mutant (ΔrpoE). After supplemental expression of RpoE in the ΔrpoE using a recombinant plasmid (pBADrpoE), we found that FljB:z66 expression was RpoE dependent in osmotic stress conditions by quantitative real-time PCR (qRT-PCR) and western blotting analysis. Based on the information above, we suggest that during hyperosmotic stress RpoE may promote FljB:z66 expression in S. Typhi through FliA.
Materials and Methods
Bacterial Strains and Plasmids
Salmonella enterica serovar Typhi GIFU10007, a z66-positive wild-type strain, was used in this study. Mutants and plasmids used in this study were listed in Table 1. Bacteria were cultured at 37°C in Luria–Bertani (LB) broth with 50 mM of NaCl to simulate low environmental osmolarity. For osmotic stress, NaCl was added into cultures to a final concentration of 300 mM.
Construction of the rpoE Deletion Mutant
To generate the rpoE deletion mutant strain, specific primer pairs F1A/B and F2A/B were designed and used to amplify the fragments F1 (681 bp) and F2 (369 bp) located upstream and downstream of the rpoE gene, respectively. Primers used in this study were shown in Table 2. A BamHI site was added to the 5′-termini of primers F1A and F2B, and a BglII site was added to the 5′-termini of primers F1B and F2A. F1 and F2 were amplified from a wild-type strain of S. Typhi, digested with BglII and ligated using a DNA Ligation Kit Ver.2 (TaKaRa) to generate an rpoE defective homologous fragment in which 288 bp of the rpoE gene was absent. The fragment was inserted into the BamHI site of the suicide plasmid pGMB151. The wild-type strain was transformed with the recombinant suicide plasmid by electroporation. The mutant strain was selected by PCR with primers F1A and F2B, as described previously [10], verified by sequencing rpoE gene, and designated as the mutant strain ΔrpoE.
Measurement of Bacterial Growth Under Hyperosmotic Stress Conditions
Bacteria from a single colony were inoculated into 1 ml of LB broth containing 50 mM of NaCl and incubated at 37°C overnight. Each 300 μl of culture was transferred into 30 ml of pre-warmed (37°C) fresh LB broth containing 300 mM of NaCl followed by incubation with shaking (200 rpm) at 37°C for 24 h. Growth was measured per hour using a BioPhotometer (Eppendorf). The experiment was repeated three times.
Motility Assay
Bacterial strains were grown overnight at 37°C without agitation on LB broth. Each 4 μl of culture was inoculated into the center of a 0.3% LB agar plate, which was containing 300 mM of NaCl. The plates were incubated at 37°C for 8 h, and motility was assessed qualitatively by examining the circular swim formed by the growing motile bacterial cells. The experiment was repeated three times and Student’s t-test was used to assess statistically significant differences between the groups.
RNA Extraction and DNA Microarray Analysis
Wild-type and ΔrpoE strains were grown in 1 ml of LB broth (pH 7.0) containing 50 mM NaCl at 37°C with shaking (200 rpm) overnight. Each 200 μl of cultures were then transfer-incubated in fresh 20 ml of LB broth with the same osmolarity to log phase (0.5 OD at 600 nm). To simulate hyperosmotic stress, NaCl was added in cultures to a final concentration of 300 mM, and the bacteria were incubated with shaking at 37°C for 30 min. An RNeasy kit (mini-column, QIAGEN) was used to extract the total RNA according to manufacturer’s instructions. The quantity and quality of the extracted RNA were determined by agarose gel electrophoresis and ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, USA). The extracted RNA was treated with 1 U of RNase-free DNase I (TaKaRa) at 37°C for 10 min to remove traces of DNA, and then incubated at 85°C for 15 min to inactivate the DNase. cDNA probes were synthesized using 20 μg of RNA. A genomic DNA microarray designed for S. Typhi Ty2 was used in this study. The fluorescence labeling of cDNA probe, hybridization, microarray scanning, and data analysis were performed as described previously [22]. Significant changes of gene transcription level were identified with SAM software [26]. In this study, the significant differential expression was determined by the value of log2 ratio of Cy3/Cy5 intensity which was larger than 1 or smaller than −1.
Supplemental Expression of rpoE in the rpoE Mutant
Primers P-rpoE-A and P-rpoE-B, specific to upstream and downstream regions of the gene rpoE (Table 2), were used to amplify a promoterless gene rpoE with pfu DNA polymerase (Fermentas). An NcoI site and a SalI site were added to the 5′-termini of primers P-rpoE-A and P-rpoE-B, respectively. An approximately 600 bp amplicon was inserted into the NcoI and SalI sites of the expression vector pBAD/gIII (Invitrogen) to form the recombinant plasmid (pBADrpoE). The positive plasmid was verified by digestion with NcoI and SalI and sequence analysis. The ΔrpoE was transformed with pBADrpoE and designated as ΔrpoE(pBADrpoE). As a control, the ΔrpoE was also transformed with the empty vector pBAD/gIII and designated as ΔrpoE(pBAD). Expression of rpoE in ΔrpoE(pBADrpoE) was induced by l-arabinose (0.2% w/v).
Quantitative Real-Time PCR
Bacteria were cultured as described above for DNA microarray analysis. Total RNA was extracted at 30 and 120 min of the hyperosmotic stress. Specific primers used for qRT-PCR are shown in Table 2. cDNA was generated using 2 μg of total RNA and 1 μg random nonamer primers. Each 1 μl of reverse transcriptional product was subjected to the qRT-PCR assay as described in the previous method [11]. To ensure that there was no contamination of genomic DNA, negative controls were performed using cDNA generated without reverse transcriptase as templates. Reactions containing primer pairs without a template were also included as blank controls. A standard curve was made for each RNA preparation. Relative transcriptional level was determined by calculating the threshold cycle (ΔC t) of each gene with RNA isolated from bacteria cells as starting material. RNA extraction, reverse transcription, and real-time PCR were performed with three independent samples in duplicate.
Extraction of Secreted Proteins and Western Blotting for FljB:z66
The wild-type strain and mutant strains ΔrpoE, ΔrpoE(pBAD), and ΔrpoE(pBADrpoE) were precultured in LB broth containing 50 mM NaCl overnight and recultured with the fresh LB broth with the same concentration of NaCl to log phase (0.5 OD at 600 nm). NaCl was added to cultures to final 300 mM for hyperosmotic stress as above. At 30 and 120 min of the stress, each 10 ml of cultures was taken for extraction of secreted proteins. Extraction and separation of the secreted proteins with SDS-PAGE were carried out according to the previously described method [10]. Identical gels were run in parallel and stained with Coomassie blue to confirm equal loading. For immunoblotting, proteins separated by SDS-PAGE were electrotransferred to PVDF membranes (Bio-Rad) at 350 mA for 150 min in the buffer containing 48 mM Tris–HCl (pH 8.3), 38.6 mM glycine, 20% (v/v) methanol, and 0.037% SDS. Membranes were incubated in 0.1 M Tris-buffered saline (TBS) buffer (pH 7.4) containing 5% skim milk at 37°C for 4 h. After washing with 0.1 M TBS buffer (pH 7.4) containing 0.02% Tween-20 (TBS-T) three times, for 10 min each, membranes were incubated at 37°C for 2 h with rabbit anti-z66 antiserum (National Institute of Infectious Disease, Japan) diluted with 0.1 M phosphate buffered saline (PBS) pH 7.4 at 1:2000. Membranes were washed with TBS-T three times, for 10 min each, and treated with goat anti-rabbit IgG (γ-chain specific) antibody conjugated with peroxidase (Boster, Wuhan, China) 1:4000 in 0.1 M PBS. After incubation at 37°C for 2 h, membranes were washed three times with TBS-T and blots developed with the DAB detection kit (Boster) according to manufacturer’s instructions.
Results
Deletion of rpoE Affects Motility and Growth in Hyperosmotic Conditions
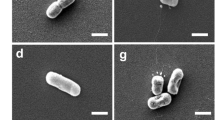
The rpoE mutant was confirmed by PCR using specific primers to the upstream and downstream regions of the rpoE gene and DNA sequencing of the PCR product. The sequencing result showed that 288 bp of the rpoE gene was successfully deleted in the ΔrpoE. The growths of S. Typhi strains were measured. Under hyperosmotic stress conditions, the rpoE mutant grew more slowly than the wild-type strain, and the defection was restored in ΔrpoE(pBADrpoE) (Fig. 1). While the growth of ΔrpoE strain and the wild-type strain at low osmotic conditions had no significant difference (data not shown). So, high osmotic stress may be the necessary stimulon for RpoE to perform its role on the growth of S. Typhi. Whether deletion of rpoE affects the motility was assessed by comparing the wild-type strain with ΔrpoE. After 8 h of incubation on motility agar plates, the motility of ΔrpoE was greatly decreased in comparison with the wild-type parental strain, and the defection was restored in ΔrpoE(pBADrpoE) (Fig. 2). These results revealed that RpoE was required for motility of S. Typhi and also suggested that RpoE could act as a modulator on S. Typhi motility rates and/or chemotaxis.
Expression of fliA and Class-2 and Class-3 Flagellar Genes is Decreased in the rpoE Mutant
Salmonella enterica serovar Typhi genomic DNA microarray was used to investigate the effect of RpoE on gene expression, especially on transcriptional expression differences in flagellar- and chemotaxis-associated genes in the wild-type and ΔrpoE strains. The results are shown in Table 3. Most of the class-3 flagellar genes and a few of the class-2 flagellar genes, including fliA encoding an RNA polymerase sigma factor for expression of the flagellar operon, were downregulated in ΔrpoE after 30 min of hyperosmotic stress. However, no obvious regulation differences were observed for flhDC, the global flagellar transcriptional activator genes. Class-3 flagellar genes are regulated by FliA, but some flagellar genes are both class-2 and class-3, such as flgKL. Transcription of flgKL is switched on both class-2 and class-3 promoters. The class-2 promoter of the operon flgBCDEFGHIJKL is regulated by a complex of FlhDC and σ70, while the class-3 promoter in operon flgKL is regulated by FliA. Our microarray analysis result showed that class-2 promoters were unchanged but class-3 promoters were obviously downregulated in the ΔrpoE. A similar phenomenon was observed for flgMN, suggesting that RpoE may promote the transcription of fliA but not flhDC in the early stage of hyperosmotic stress in S. Typhi.
RpoE Affects Transcriptional Expression of fliA and fljB:z66 But Not flhD at 30 and 120 min Under Hyperosmotic Stress
To verify whether or not the expression of the global flagellar regulator FlhDC is affected by RpoE during hyperosmotic stress, qRT-PCR was performed. The result revealed that no obvious expression difference of flhD was observed in ΔrpoE compared to the wild-type strain upon hyperosmotic stress for 30 and 120 min, which indicated that RpoE did not initiate the transcription of flhDC during hyperosmotic stress (Fig. 3a). To further investigate the effect of RpoE on flagellar gene expression, the mutant strain ΔrpoE was transformed with the recombinant expression plasmid pBADrpoE, and transformed with the empty vector pBAD for control. qRT-PCR was performed to detect the transcriptional expression of the regulator gene fliA (Fig. 3b) and the flagellin gene fljB:z66 (Fig. 3c). The results showed that the expression of fliA and fljB:z66 in ΔrpoE compared to the wild-type was reduced about five and eightfold at 30 min of hyperosmotic stress, respectively. The reduction became more obvious at 120 min of hyperosmotic stress. The results indicated that RpoE affected the transcriptional expression of fliA and fljB:z66, but not flhD at 30 and 120 min of hyperosmotic stress.
qRT-PCR was performed to detect the transcriptional expression difference of flhD, fliA, and fljB:z66 between wild-type and the rpoE mutant at 30 and 120 min of hyperosmotic stress. Data are the mean ± SD from three independent experiments. a Expression of flhD in ΔrpoE appeared no significant difference compared to the wild-type strain at 30 and 120 min of hyperosmotic stress. b After treated for 30 min upon hyperosmotic stress, fliA mRNA decreased about fivefold in the rpoE mutant compared to the wild-type strain. The reduction of fliA in ΔrpoE became more obvious at 120 min of hyperosmotic stress. c After treated for 30 min upon hyperosmotic stress, fljB:z66 mRNA reduced eightfold in ΔrpoE compared to wild-type strain. This phenomenon also became more obvious at 120 min of hyperosmotic stress. d The expression of the known rpoE-dependent gene htrA also changed in the microarray assay as control
FljB:z66 Expression Requires RpoE Under Hyperosmotic Stress
Monomeric flagellin is synthesized by Salmonella, secreted through a central channel in the flagellar basal body and hook, and polymerized to form a flagellum. Flagellin can be detected in proteins secreted by Salmonella. To further investigate the regulation of RpoE on fljB:z66 in S. Typhi, the expression of the FljB:z66 protein in ΔrpoE was investigated. FljB:z66 expression was detected by western blotting (Fig. 4). The results showed that FljB:z66 expression was obviously decreased in ΔrpoE compared to the wild-type strain. The level of FljB:z66 was restored in ΔrpoE(pBADrpoE), but not in the control strain ΔrpoE(pBAD). The defective expression of FljB:z66 in ΔrpoE after high osmotic stress was more obvious at 120 min than at 30 min. These results indicated that the expression of fljB:z66 might be promoted by the RpoE protein in S. Typhi.
Western blot analysis was performed to detect FljB:z66 expression. At 30 min of hyperosmotic stress, the expression of FljB:z66 in ΔrpoE was decreased compared to the wild-type strain. The expression of FljB:z66 was restored in ΔrpoE (pBADrpoE), but still maintained a low level in the empty-vector control ΔrpoE (pBAD). Expression of FljB:z66 in ΔrpoE at 120 min of hyperosmotic stress was more depressed than at 30 min
Discussion
Salmonella enterica serovar Typhi is a gram-negative, enteroinvasive pathogen that is the cause of typhoid fever. Infection is initiated when bacteria enter the gastrointestinal tract from contaminated water or food. When bacteria arrive at the distal ileum, they enter the specialized intestinal epithelial M cells of Peyer’s patches. Upon invading the intestine, the pathogen migrates into the mesenteric lymph nodes and reaches the liver, spleen, and bone marrow through the blood and lymph systems, where they replicate and cause systemic infection [6]. Intestinal invasion by Salmonella is associated with many pathogen-specific factors, such as flagella and motility [12, 16]. Most invasion factors of Salmonella are affected by the osmolarity of the ambient environment [15, 24]. Once S. enterica enters the intestinal lumen of the host, it is confronted with an extreme environment that includes an increase in osmolarity. The osmolarity surrounding Salmonella is approximately 50 mM NaCl in food, and approximately 300 mM in the small intestine lumen [19]. Therefore, the regulation of Salmonella in hyperosmotic environments is important for invasion through the small intestine.
The expression of flagella-related genes is affected by environmental factors, such as osmotic [23]. FlhDC is the global regulator of flagellar and motility-related genes. FliA is a class-2 flagellar gene product promoted by FlhDC, can activate expression of most class-3 flagellar genes [14]. We previously found that the expression of fljB:z66 was dependent on FliA at both high and low osmolarity [28]. In addition, previous research on genomic expression kinetics during an hyperosmotic stress of S. Typhi found that the repressed expression of most flagellar and chemotaxis genes at early stage of the osmotic stress was recovered after 120 min of the stress, and suggested that S. Typhi might gradually adapted to hyperosmotic conditions and recovered the motility after 120 min [11]. When bacterial enter the enteric tract, they will submit the hyperosmotic surroundings. After 120 min the bacteria are likely in the distal ileum, so motility is necessary to invade the specialized intestinal epithelial M cells of Peyer’s patches. In this study, we compared the gene expression of a mutant ΔrpoE with a wild-type strain after osmotic stress. Our results revealed that the expression of flhD did not change, but the expression of the class-2 flagellar gene fliA and the class-3 flagellar gene fljB:z66 obviously decreased in the mutant ΔrpoE after 30 min of high osmotic stress. At 120 min, expression levels of fliA and fljB:z66 in the ΔrpoE strain decreased more obviously. These results suggest that RpoE may activate flagellar gene expression through the class-2 flagellar gene fliA, but not the class-1 flagellar genes during hyperosmotic stress.
RpoE can be released from RseA located on the inner membrane under osmotic stress [21]. We previously found that the expression of rpoE decreased at the early stage of stress and was restored at a later stage in wild-type strain [11]. The expression kinetics of fljB:z66 and many flagellar genes were similar to rpoE. At a later stage of osmotic stress, the high expression and release of RpoE will increase the amount of free RpoE in the cytoplasm and then promote the expression of downstream genes. We speculate that under hyperosmotic stress, S. Typhi may increase the flagellar expression and motility through promoting fliA expression by RpoE. It may be a new regulatory pathway that S. Typhi increase invasive ability in distal ileum, and that needs further research to be clarified.
Recent experiments demonstrated that the flagellum itself can be a sensor. For example, suboptimal external surface hydration was shown to interfere with secretion of flagellin subunits and filament growth [27]. Additionally, the flagella of S. enterica serovar Typhimurium can sense a shift in environmental temperature and modulate flagella motility through conformational changes of the flagellar switch component FliG [17]. Recently, by microarray analysis, we found that 122 genes were upregulated and 65 genes were downregulated by more than threefold in z66-antiserum-treated samples [29]. Such broad changes in gene regulation were not observed in a z66 negative strain under similar conditions. This study provided more evidence to support the hypothesis that flagella can sense extracellular antibody and regulate gene expression. Considering that the flagellum itself can be a sensor, we speculated that RpoE might play a partial role in response to cell envelope stress, by regulating flagella.
In summary, our results indicated that, under hyperosmotic conditions, RpoE can regulate FljB:z66 expression and it may be initiated from fliA expression in S. Typhi and may be beneficial to invasion. This finding may help us to understand the regulation of gene expression of S. Typhi in response to enteric hyperosmotic conditions.
References
Alba BM, Gross CA (2004) Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol 52:613–619
Alba BM, Leeds JA, Onufryk C et al (2002) DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev 16:2156–2168
Alba BM, Zhong HJ, Pelayo JC, Gross CA (2001) degS (hhoB) is an essential Escherichia coli gene whose indispensable function is to provide sigma (E) activity. Mol Microbiol 40:1323–1333
Baker S, Hardy J, Sanderson KE et al (2007) A novel linear plasmid mediates flagellar variation in Salmonella Typhi. PLoS Pathog 3:e59
Baker S, Holt K, Whitehead S et al (2007) A linear plasmid truncation induces unidirectional flagellar phase change in H:z66 positive Salmonella Typhi. Mol Microbiol 66:1207–1218
Everest P, Wain J, Roberts M et al (2001) The molecular mechanisms of severe typhoid fever. Trends Microbiol 9:316–320
Frankel G, Newton SM, Schoolnik GK, Stocker BA (1989) Intragenic recombination in a flagellin gene: characterization of the H1-j gene of Salmonella typhi. EMBO J 8:3149–3152
Grigorova IL, Chaba R, Zhong HJ et al (2004) Fine-tuning of the Escherichia coli sigmaE envelope stress response relies on multiple mechanisms to inhibit signal-independent proteolysis of the transmembrane anti-sigma factor, RseA. Genes Dev 18:2686–2697
Hayden JD, Ades SE (2008) The extracytoplasmic stress factor, sigmaE, is required to maintain cell envelope integrity in Escherichia coli. PLoS ONE 3:e1573
Huang X, Phung le V, Dejsirilert S et al (2004) Cloning and characterization of the gene encoding the z66 antigen of Salmonella enterica serovar Typhi. FEMS Microbiol Lett 234:239–246
Huang X, Xu H, Sun X et al (2007) Genome-wide scan of the gene expression kinetics of Salmonella enterica serovar Typhi during hyperosmotic stress. Int J Mol Sci 8:116–135
Jones BD, Lee CA, Falkow S (1992) Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun 60:2475–2480
Kutsukake K, Iino T (1994) Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J Bacteriol 176:3598–3605
Kutsukake K, Ohya Y, Iino T (1990) Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol 172:741–747
Leclerc GJ, Tartera C, Metcalf ES (1998) Environmental regulation of Salmonella typhi invasion-defective mutants. Infect Immun 66:682–691
Liu SL, Ezaki T, Miura H et al (1988) Intact motility as a Salmonella typhi invasion-related factor. Infect Immun 56:1967–1973
Mashimo T, Hashimoto M, Yamaguchi S, Aizawa S (2007) Temperature-hypersensitive sites of the flagellar switch component FliG in Salmonella enterica serovar typhimurium. J Bacteriol 189:5153–5160
Miticka H, Rowley G, Rezuchova B et al (2003) Transcriptional analysis of the rpoE gene encoding extracytoplasmic stress response sigma factor sigmaE in Salmonella enterica serovar Typhimurium. FEMS Microbiol Lett 226:307–314
Pickard D, Li J, Roberts M et al (1994) Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun 62:3984–3993
Rhodius VA, Suh WC, Nonaka G et al (2006) Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol 4:e2
Rolhion N, Carvalho FA, Darfeuille-Michaud A (2007) OmpC and the sigma(E) regulatory pathway are involved in adhesion and invasion of the Crohn’s disease-associated Escherichia coli strain LF82. Mol Microbiol 63:1684–1700
Sheng X, Huang X, Mao L et al (2009) Preparation of Salmonella enterica serovar Typhi genomic DNA microarrays for gene expression profiling analysis. Prog Biochem Biophys 36:306–312
Shin S, Park C (1995) Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol 177:4696–4702
Tartera C, Metcalf ES (1993) Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun 61:3084–3089
Testerman TL, Vazquez-Torres A, Xu Y et al (2002) The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol 43:771–782
Tusher V, Tibshirani R, Chu C (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5512
Wang Q, Suzuki A, Mariconda S et al (2005) Sensing wetness: a new role for the bacterial flagellum. EMBO J 24:2034–2042
Xu S, Zou X, Sheng X et al (2010) Expression of fljB:z66 on a linear plasmid of Salmonella enterica serovar Typhi is dependent on FliA and FlhDC and regulated by OmpR. Braz J Microbiol (accepted)
Zhang H, Sheng X, Xu S et al (2009) Global transcriptional response of Salmonella enterica serovar Typhi to anti-z66 antiserum. FEMS Microbiol Lett 298:51–55
Zou X, Huang X, Xu S et al (2009) Identification of a fljA gene on a linear plasmid as the repressor gene of fliC in Salmonella enterica serovar Typhi. Microbiol Immunol 53:191–197
Acknowledgments
We thank T. Ezaki (Gifu University) for bacterial strains, anti-z66 antiserum, and continuous support. This study was supported by National Natural Science Foundation of China (30870095), National Special Scientific Program (2008ZX10004-009), Science Foundation of Jiangsu University for Advanced Scholars and Special Team Program of Jiangsu University (2008-018-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, H., Sheng, X., Zhang, H. et al. RpoE may Promote Flagellar Gene Expression in Salmonella enterica Serovar Typhi Under Hyperosmotic Stress. Curr Microbiol 62, 492–500 (2011). https://doi.org/10.1007/s00284-010-9734-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9734-y