Abstract
Xylanase is one of the most important hemicellulases in industry. However, its low thermostability limits its applications. In this study, one thermostable xylanase-producing strain 400264 was obtained from screening 11 Aspergillus niger strains (producing thermotolerant xylanase), and the optimum temperature of crude xylanase extracted from it was 55°C. Original activity of the crude xylanase is 64% at 60°C and 55% at 85°C with an incubation time of 30 min, respectively. After the expression of recombinant xylanase gene (xynA/xynB), the XYNB (xylanase B) showed higher thermostability than XYNA (xylanase A). Recombinant enzyme XYNB retained 94% of its activity for 10 min at 85°C, while XYNA with no activity left. Site-directed mutagenesis was performed to replace Ala33 of XYNB by Ser33 resulting 19% decrease in enzyme activity after incubating at 85°C for 30 min. It suggested that the Ala33 residue may have a certain effect on the thermophilic adaptation of xylanase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xylan is the second largest renewable resource in nature. The complete degradation of xylan requires the participation of a variety of enzymes. Xylanases (EC3.2.1.8) are glycosyl hydrolases that degrade the linear polysaccharide β-1,4-xylan into xylose [1], which are believed to have considerable potential in various industrial applications, such as in food, animal feed, textile, and paper industries [2]. All of the industries require high-temperature processes, but the thermostability of the native strain is rare, so improving the thermophilic adaption of xylanase is very important.
Currently, there are two approaches that have been successfully tried to obtain thermophilic adaption of xylanase. One approach is to screen thermophilic adaption strains from natural microorganisms and the other is to use of genetic engineering on enzyme molecules through in vitro transformation [3]. Nowadays, different forms of xylanase have been isolated and characterized from various microbial sources, which are alkaliphilic, thermophilic, or acidophilic [4–6]. However, to further increase the potentials of xylanase in industrial applications, some researchers have transformed of xylanase to study its thermostability. Through site-directed mutagenesis, the conserved residue is an essential amino acid for the thermostability of xylanase [7] and in addition, engineering multiple amino acids such as arginines into the Ser/Thr surface increased the thermotolerance and shifted the pH optimum towards alkalinity [8]. The thermostability and specific activity are both enhanced via mutation of the interior hydrophobic region of the enzyme by directed evolution and site-directed mutagenesis to screen thermostable mutants of a family of 11 xylanases [9].
Xylanases from Aspergillus niger are secretory [10] and acid-resistant proteins [11], and belong to family G/11 of glycosyl hydrolase. xynA and xynB are the main genes encoding xylanase reported in A. niger, but have a non-identical sequence [12]. Xylanase from different species vary greatly in amino acid composition; however, the compositions of their catalytic regions are more consistent, while the enzymes of the same family have high sequence homology. There is no homology of catalytic site between XYNA/XYNB and cellulase, indicating that the two enzymes evolved from different ancestry, or some may contain more than one catalytic site. The amino acid composition of xylanase and cellulase active centers are similar, whereby tryptophan and carboxyl groups are the main residues, with some also containing cysteine residues, involved in substrate-binding or catalyzing [13]. During evolution, the residues, which are very important to the molecular construction and catalytic activities of enzyme, are highly conserved.
In this present research, we isolated and screened 11 A. niger strains producing xylanases with higher thermostability. Yi et al. cloned the xylanase encoding gene xynA and xynB from A. niger 400264 and compared the thermotolerance of the xylanase XYNA and XYNB, and found that recombinant XYNB had increased thermophilic adaption, much greater than the recombinant enzyme XYNA, but its thermophilic adaption mechanism remains unclear (Yi et al., unpublished data). We aligned xynB (FJ 772090) with reported A. niger xynB (AY126481, AF490982, AY551187, AY 536639) in this study, and found only one amino acid (residue 33) difference in the mature peptide. Here, residue 33 corresponds to Ala, while the corresponding site in reported A. niger xynB is Ser, thus Ala33 may have a certain role in the maintenance of thermophilic adaptation of A. niger xynB. Through site-directed mutagenesis of the Ala33 residue of XYNB, we explored the role of residues 33 in thermostability.
Materials and Methods
Strains and Reagents
11 Aspergillus niger strains isolated from soil were named as 400284, 400095, 400354, 400406, 400311, 400317, 400323, 400078, 400321, 400083, 400264, respectively. Escherichia coli strains (JM109 and BL21 (DE3) and 3 plasmid vectors (pET32a, pMD-xynA, pMD-xynB) were from our own laboratory.
Xylan from oat spelts (Sigma Company); 3′ Full race kit, EX Taq DNA polymerase, PyroBest DNA polymerase, Primer STARTMHS DNA Polymerase, restriction endonucleases (EcoRI and XholI), were all purchased from TaKaRa; E.Z.N.A. TM Gel Extraction Kit(Omega Company); Sodium dodecyl sulfate (SDS), Tris, bovine serum albumin (BSA), thalidomide acrylamide, N,N′-methylenebisacrylamide, N,N,N′,N′-2-tetramethylethylenediamine (TEMED), were imported and subpackaged; the other agents were analysis purified.
Enzyme Assay (Determination of Enzyme Activity and Enzymatic Properties)
Xylanase activity was quantified using 3,5-dinitrosalicylic acid (DNS) method as described by Bailey et al. [14]. Pure xylose was used as the standard reducing sugar to generate the standard curve. One unit (IU) of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol of reducing xylose per minute. According to this method, xylanase activities of extracts were determined under different conditions (temperature, pH, metal ions).
Screening of Strains Producing Thermotolerant Xylanase
Aspergillus niger strains were cultured in PDA medium (potato, 20%; glucose, 2%; KH2PO4, 0.3%; MgSO4·7H2O, 0.25%; agar, 1.5%). Single-spore suspensions were made when the mediums was covered fully with spores, then the strains were grown by solid-state fermentation using a medium based on a mixture of rice bran and rice husk (1:1 on a dry weight basis) with a initial dry matter concentration of 3 × 105 CFU/g, at 30°C. After 48 h, we determined xylanase activity of different strains, respectively. Crude enzymatic extracts from different strains were treated at 85°C, after 30 min they were quickly cooled and measured for remaining xylanase activity. We could thus screen the best strain which produced xylanase with high thermotolerance and enzymatic activity.
Construction of Expression Plasmid
Xylanase was expressed in E. coli. PCR was performed by using the primers of xynA-P1 (5′-GGA ATT CAG TGC CGG TAT CAA CTA CGT G-3′) and xynA-P2 (5′-CCG CTC GAG TTA AGA GGA GAT CGT GAC ACT GG-3′), and plastid pMD-xynA was used as template to obtain the xynA gene fragment without the signal sequence. In the same way, we obtained xynB gene fragment by using primers of xynB-P1 (5′-GGA ATT CTC GAC CCC GAG CTC GAC CGG CGA GAA-3’) and xynB-P2 (5′-CCG CTC GAG TTA CTG AAC AGT GAT GGA GGA AGA-3′). The two amplified fragments were digested with EcoRI and XholI and ligated into pET-32a vector previously digested with the same enzymes, the recombinant pET32a-xynA and pET32a-xynB plasmids were transformed into E. coli strain JM109.
Expressions and Purification of Xylanase from E. coli
Recombinant plasmids were introduced into E. coli strain BL21 (DE3) and transformants were selected on LB plates supplemented with ampicillin (50 μg/ml). Culture of E. coli cells (A600 0.6–0.8) was induced by 1 mM IPTG and grown for 4 h. Cells were harvested by centrifugation, disrupted by sonication, and the supernatant was purified by using nickel-affinity chromatography (Qiagen). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the Laemmli method [15], the separating gel, and stacking gel concentrations were 12 and 5%, respectively. Coomassie brilliant blue R-250 staining was used to stain protein Ni affinity chromatography purification: Ni-chelating metal affinity chromatography column (2 cm × 8 mm) and medium Chelating HP chromatography were purchased from Amersham Phamacia Company. The Bradford Protein Assay Kit was used to determine the protein content against a BSA standard curve.
Site-Directed Mutagenesis of the xynB Gene
Amino acid sequence alignment was performed between xynB and A. niger xynB (AY126481, AF490982, AY551187, AY536639), and determined that the mutant site was Ala33Ser. The mutations were generated by the overlap extension PCR method, in which the mutations were introduced into the oligonucleotide primers. PCR was performed using the primers of xynB-R (5′-GGA ATT CTC GAC CCC GAG CTC GAC CGG CGA GAA-3’), antisense TB (5′-ACT CAA CAG TGT AGG AAC CAG CAT CTC-3′), sense TB (5′-CCA ACG GAG ATG CTG GTT CCT ACA CTG-3′) and xynB-antisense (5′-CCG CTC GAG TTA CTG AAC AGT GAT GGA GGA AGA-3′). Two recovered fragments were used as templates and primers for each other for three cycles of PCR, then by adding primers sense B and antisense B, we obtained the full-length xynB mutant gene (without signal peptide sequence). The products of TA cloning were transformed into competent E. coli JM109, and by use of bacterial liquid PCR and double restriction enzyme digestion, we verified the positive clones, and sequenced.
Results
Screening of A. niger Strains Producing Thermostable Xylanase
The thermostabilities of crude enzymatic extracts of 11 A. niger strains were measured. All crude enzyme extracts were treated at 85°C for 30 min, and then determined for their relative xylanase activity. Crude enzymatic extract from A. niger 400264 had high enzyme activity of 920 U/g, and also retained 55% of its original activity post-treatment.
Temperature Optimum and Thermophilic Adaption
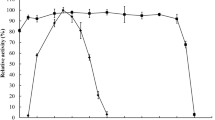
The optimum temperature of crude xylanase from A. niger 400264 was 55°C (Fig. 1a). After treated at 50°C for 30 min, the activity of crude enzymatic extract was almost as high as before; crude enzyme showed 64 and 55% relative activity after incubating for 30 min at 60 and 85°C, respectively (Fig. 1b).
Characteristics of crude xylanase and thermostability of XYNA and XYNB from A. niger 400264. a The optimum temperature of crude xylanase (filled diamond), b the thermostability of crude xylanase treated 10 min at different temperatures (filled diamond), c the optimum pH (filled triangle) and pH stability (filled square) of crude xylanase, d the thermostability of xynA (filled triangle), xynB (open triangle) treated different time in 85°C
pH Optimum and Stability of Xylanase from A. niger 400264
Xylanase from A. niger 400264 has high pH stability [16], with the optimum pH at 5.0 (Fig. 1c). The enzyme showed above 90% of xylanase activity in a pH range of 3.0–7.0, and 80% at pH was 8.0.
Effect of Metal Ions on the Activity of Xylanase from A. niger 400264
The chemical composition based on the presence or absence of some metal ions (the final concentration was 1 mmol/l) greatly influenced the activity of xylanase. K+, Mg2+, Ca2+, Fe2+ enhanced the enzymatic activity; and Co2+, Zn2+, Fe3+ had almost no effect on its activity; while Mn2 and Cu2+ inhibited enzymatic activity.
Thermotolerant Comparison Between XYNA and XYNB from A. niger 400264
Recombinant XYNA and XYNB were expressed in E. coli and purified, analysis of SDS-PAGE (Fig. 2) showed that the XYNA and XYNB produced a specific clear protein band and the molecular mass were about 41 kDa, respectively. The specific activities of XYNA and XYNB had reached to 16.58λ·(U mg)−1 and 1201.7λ·(U mg)−1, respectively (Table 1). XYNB retained 94% of the activity after incubation for 10 min at 85°C, while the activity of recombinant enzyme XYNA was completely lost (Fig. 1d). It suggests that XYNB is better to perform further experiments.
Site-Directed Mutation of Ala33 and Properties of Mutant XYNB from A. niger 400264
The kinetic parameters of mutant and non-mutant XYNB were determined. The K m and V max values of the mutant were 99.5 mg/ml and 5000 μmol/min mg, while the K m value of non-mutant XYNB was 18.7 mg/ml, indicating that the mutant enzyme-substrate affinity had decreased dramatically. We detected the enzymatic activities of both mutant XYNB and wild-type XYNB at different temperatures (Fig. 3a), the results showed that the optimum temperature of mutant XYNB was 50°C compared to 55°C of wild-type, and the optimum pH of mutant XYNB, exactly as the same wild-type, was 5.0. The mutant enzyme performed well between pH 4.0 and 6.0, but when the pH value exceeded this scale its activity decreased (Fig. 3b). The mutant enzyme appeared to higher pH stability compared to wild-type, especially between 5.0 and 10.0 (Fig. 3c).
Characteristics and thermostability of site-directed mutagenesis XYNB from A. niger 400264. a The optimum temperature (mutant XYNB (filled triangle), wild-type XYNB (filled square)); b the optimum pH (mutant XYNB (filled triangle), wild-type XYNB (filled square)); c pH stability (mutant XYNB (filled triangle), wild-type XYNB (filled square)); d the thermostability of mutant XYNB treated in 60°C (filled diamond), wild-type XYNB treated in 60°C (filled square), mutant XYNB treated in 85°C (filled triangle), wild-type XYNB treated in 85°C (open triangle)
Thermostability of Mutant XYNB
The thermostabilities of mutant and wild-type XYNB were determined (Fig. 3d). The thermostability of mutant enzyme dramatically decreased after incubation at both 60 and 85°C. The mutant enzyme retained 81.5% of its relative activity after incubation at 60°C for 60 min, while the wild-type retained 94% of its activity. After incubation at 85°C for 30 min, the mutant enzyme retained 60% relative activity, while the wild-type retained 79% of its activity; and after incubation at 85°C for 60 min, the mutant enzyme retained 40% of its relative activity, while the wild-type retained 53% of its activity. All the above indicated that the Ala33 residue plays an important role in the high thermotolerance of XYNB from A. niger 400264.
Tertiary Structure Prediction of A. niger XYNB
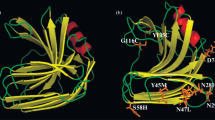
The amino acid sequence of xylanase XYNB from A.niger was put through the On-line Analysis System [17], and a homology modeling of XYNB was constructed by Swiss-Model (Fig. 4).
Discussion
Thermostability of the Family Xylanase of A. niger
Aspergillus niger xylanase XYNA and XYNB all belong to G/11 family, whose Glu, the catalytic residue of the enzyme, are very conserved. There are two conserved catalytic active at sites E79 and E170 in XYNA of the A. niger 400264, which are consistent with the reported [18]. However, it had found that the same conservative catalytic active site E121, E212 [19] in XYNB of the A. niger 400264 in consonance with reported. As to screening the strain producing thermostable xylanase, we analyzed the crude enzyme extracts, including the enzymatic activity of XYNA and XYNB, and it was difficult to tell the difference between thermostabilities of the two [20]. By expression of XYNA and XYNB from A. niger 400264 in E. coli, purification, then determination of enzymatic activity, we found the activity of XYNB higher than XYNA, and this was in consonance with reported [21, 22]. To our knowledge, the activity of the XYNB reported here is higher than those of a majority of the xylanase [4–6].The good thermostability of XYNB of A. niger 400264 shows its potential for industrial applications.
The Analysis of Thermostable Site of Xylanase of A. niger 400264
Previous studies have demonstrated that substitutions of amino acids in the certain site of some enzymes lead to the increase in the thermotolerance of the protein without causing conformational changes [8, 26]. Sriprang et al. reconstructed XYLB (AAS67299.1) of Aspergillus BCC14405, replacing Arg for Ser/Thr, and obtained two mutants ST4 and ST5, which had higher thermostability. The optimum temperature increased to 5 and 10°C, respectively, and they showed 80% enzymatic activity compared to wild type, and remaining only 15% when treated at 50°C for 2 h. As to selecting the mutable site, ST5 had one amino acid mutation more than ST4, i.e., 33Ser [23]. In this study, the protein encoded by the cloned gene of A. niger 4000264 xynB (ACN89393.1) showed only one different amino acid in contrast to XYLB. The same was the site of 33rd; the different was the amino acid in this site, in A. niger 400264 was Ala, whereas XYLB was Ser. These two recombinant enzymes showed different thermostabilities. Recombinant XYNB displayed 94% of its enzymatic activity when incubating at 60°C for 60 min, whereas the recombinant enzyme XYLB (wild type) of Sriprang et al. only remained at 20%, thus, inferring the amino acid of this site has effects on the thermostability of xylanase.
Site-Directed and Thermostable Molecular Mechanism of Xylanase Gene xynB of A. niger
Previous study has already indicated that through site-directed mutagenesis, replacing some amino acids to another, may increased the optimum temperature, pH adaption or thermophilic adaption [24–26]. In this study, through analysis of tertiary structure obtained by homology modeling, the outer anti-parallel β-sheet of both XYNB and XYLB were composed of a plane, and it affected relative motion between functional parts within the enzyme molecule due to the interactions of the plane and its water environment. In this study, the first 33 amino acids were on the plane composed of the outer anti-parallel β-sheet. According to the polarity of the nature of R-based, Ala is a non-polar R-based amino acid, while Ser belongs to non-charged polar R-based amino acid. Ser is more hydrophilic than Ala, thus it is favorable for the interaction of enzyme molecules and water molecules to undergo Brownian motion in solution, and it is unfavorable to the maintenance and integrity of the structure and spatial conformation of enzyme molecules. Moreover, the 33Ala mutation of Ser in XYNB reduces the distance between Asp29 and The185 on the flank, from 9.76 to 9.65. Meanwhile, the distance between Asp18 and Thr128 of the substrate-binding region increased from 14.10 to 14.99, and the Final Total Energy of wild type and mutant enzymes were −7948.152 kJ/mol and −7973.549 kJ/mol, respectively. The thermostability of mutant enzyme Ala33Ser is significantly reduced by site-directed mutagenesis of Ala33, and it proved that Ala33 was the crucial factor in maintaining the thermostability of the A. niger XYNB.
In conclusion, our results indicated that the change of a single amino acid has some effect on the stability of the enzyme, but a variety of factors has caused this result. The factors affecting the thermostability of xylanase are attributed to the content of Arg and Pro [27], the formation of salt bridges of protein structure [28], the number of hydrogen bonds, the introduction of disulfide bonds [29], the composition of conservative amino acid, and the interaction of aromatic amino acid [30], and so on. The specific mechanisms that alter the thermostability of A. niger 400264 XYNB still requires further study.
References
Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi properties and industrial applications. Appl Microbiol Biotechnol 67(5):577–591
Li Y, Zhang B, Chen X, Chen Y, Cao Y (2009) Improvement of Aspergillus sulphureus Endo-beta-1,4-xylanase expression in Pichia pastoris by codon optimization and analysis of the enzymic characterization. Appl Biochem Biotechnol. doi:10.1007/s12010-009-8621-0
Shanklin J (2008) Enzyme engineering. Adv Plant Biochem Mol Biol 1:29–47
Wang J, Zhang WW, Liu JN, Cao YL, Bai XT, Gong YS, Cen PL, Yang MM (2009) An alkali-tolerant xylanase produced by the newly isolated alkaliphilic Bacillus pumilus from paper mill effluent. Mol Biol Rep. doi:10.1007/s11033-009-9915-6
Yi X, Sun L, Liu X, Tao HH, Abudukerim M, Rahman E (2007) Xylanase production and properties by newly isolated from thermostable alkaliphilic Chinese Bacillus strains. Biotechnology 17(5):31–35
Annamalai N, Thavasi R, Jayalakshmi S, Balasubramanian T (2009) Thermostable and alkaline tolerant xylanase production by Bacillus subtilis isolated form marine environment. Indian J Biotechnol 8(3):291–297
Roberge M, Shareck F, Morosoli R, Kluepfel D, Dupont C (1998) Site-directed mutagenesis study of a conserved residue in family 10 glycanase:histidine 86 of xylanase A from Streptomyces licidans. Protein Eng 11(5):399–404
Turunen O, Vuorio M, Fenel F, Leisola M (2002) Engineering of multiple arginines into the Ser/Thr surface of Trichoderma reesei endo-1,4-beta-xylanase II increase the thermotolerance and shifts the pH optimum towards alkaline pH. Protein Eng 15(2):141–145
You C, Huang Q, Xue HP, Xu Y, Lu H (2009) Potential hydrophobic interaction between two cysteines in interior hydrophobic region improves thermostability of a family 11 xylanase from N. patriciarum. Biotechnol Bioeng. doi:10.1002/bit.22623
Vries DE, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65(4):497–522
Weng XY, Sun JY (2005) Construction, expression, and characterization of a thermostable xylanase. Curr Microbiol 51(3):188–192
Krisana A, Rutchadaporn S, Jarupan G, Lily E, Sutipa T, Kanyawim K (2005) Endo-1, 4-xylanase B from Aspergillus cf. niger BCC14405 isolated in Thailand: purification, characterization and gene isolation. J Biochem Mol Biol 38(1):17–23
Kavya V, Padmavathi T (2009) Optimization of growth conditions for xylanase production by Aspergillus niger in solid state fermentation. Pol J Microbiol 58(2):125–130
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
LaemmLi UK (1970) Cleavage of structural proteins during the assemble of the head of bacteriophage T4. Nature 227:680–685
Bai YG, Wang JS, Zhang ZF, Yang PL, Shi PJ, Luo HY, Meng K, Huang HQ, Yao B (2010) A new xylanase from thermoacidophilic Alicyclobacillus sp. A4 with broad-range pH activity and pH stability. J Ind Microbiol Biotechnol 37(2):187–194
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Krengel U, Dijkstra BW (1996) Three-dimensional structure of endo-1, 4-β-xylanase I from Aspergillus niger: molecular basis for its low pH optimum. J Mol Biol 263:70–78
Luttig M, Pretorius IS, Van Zyl WH (1997) Cloning of two β-xylanase-encoding genes from Aspergillus niger and their expression in Saccharomyces cerevisiae. Biotechnol Lett 19(5):411–415
Luo HY, Wang YR, Li J, Wang H, Yang J, Yang YH, Huang HQ, Fan YL, Yao B (2009) Cloning, expression and characterization of a novel acidic xylanase, XYL11B1, from the acidophilic fungus Bispora sp. MEY-1. Enzyme Microb Technol 45(2):126–133
Cao YH, Chen XL, He PL, Lu WQ (2006) Cloning, expression and enzyme characterization analysis of A. sulphureus xylanase gene xynA. Lett Biotechnol 17(6):878–881
Van Ooyen AJJ, De Graaff LH, Van Den Broeck HC, Visser J (1994) Cloning and expression of xylanase B. International Patent Application WO94/14965 Gist-Brocades Delft
Sriprang R, Asano K, Gobsuk J, Tanapongpipat S, Champreda V, Eurwilaichitr L (2006) Improvement of thermostability of fungal xylanase by using site-directed mutagenesis. J Biotechnol 126(4):454–462
Moreau A, Shareck F, Kluepfel D, Morosoli R (1994) Increase in catalytic activity and thermostability of the xylanase A of Streptomyces lividans 1326 by site specific mutagenesis. Enzyme Microb Technol 16(5):420–424
Al Balaa B, Brijs K, Gebruers K, Vandenhaute J, Wouters J, Housen I (2009) Xylanase XYL1p from Scytalidium acidophilium: site-directed mutagenesis and acidophilic adaption. Bioresour Technol 100(24):6465–6571
Liu LW, Wang B, Chen HG, Wang SY, Wang MD, Zhang SM, Song AD, Shen JW, Wu K, Jia XC (2009) Rational pH-engineering of the thermostable xylanase based on computational model. Process Biochem 44:912–915
Payan F, Flatman R, Porciero S, Williamson G, Juge N, Roussel A (2003) Structural analysis of xylanase inhibitor protein I (XIP-I), a proteinaceous xylanase inhibitor from wheat (Triticum aestivum, var. Soisson). Biochemical J 372:399–405
Natesh R, Manikandan K, Bhanumoorthy P, Viswamitra MA, Ramakumar S (2003) Thermostable xylanase from Thermoascus aurantiacus at ultrahigh resolution (0.89 A) at 100 K and atomic resolution (1.11 A) at 293 K refined anisotropically to small molecule accuracy. Acta Crystallogr Sect D 59:105–117
Davoodi J, Wakarchuk WW, Carey PR, Surewicz WK (2007) Mechanism of stabilization of Bacillus circulans xylanase upon the introduction of disulfide bonds. Biophys Chem 125:453–461
Malik A, Ahmad S (2007) Sequence and structural features of carbohydrate binding in proteins and assessment of predictability using a neural network. BMC Struct Biol 7(1):1–14
Acknowledgments
This work was supported by the National Special Basic Research Projects of China (Grant No. SB2007FY400-4), by the National Basic Research Program (973) of China (Grant No. 2009CB125910).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, J., Song, L., Li, X. et al. Site-Directed Mutagenesis and Thermostability of Xylanase XYNB from Aspergillus niger 400264. Curr Microbiol 62, 242–248 (2011). https://doi.org/10.1007/s00284-010-9697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9697-z