Abstract
Current studies have indicated the utility of photodynamic therapy using porphyrins in the treatment of bacterial infections. Photoactivation of porphyrins results in the production of singlet oxygen (1O2) that damages biomolecules associated with cells and biofilms, e.g., proteins, polysaccharides, and DNA. The effect of a cationic porphryin on P. aeruginosa PAO1 biofilms was assessed by exposing static biofilms to 5,10,15,20-tetrakis(1-methyl-pyridino)-21H,23H-porphine, tetra-p-tosylate salt (TMP) followed by irradiation. Biofilms were visualized using confocal laser scanning microscopy (CLSM) and cell viability determined using the LIVE/DEAD BacLight viability assay and standard plate counts. At a concentration of 100 μM TMP, there was substantial killing of P. aeruginosa PAO1 wild-type and pqsA mutant biofilms with little disruption of the biofilm matrix or structure. Exposure to 225 μM TMP resulted in almost complete killing as well as the detachment of wild-type PAO1 biofilms. In contrast, pqsA mutant biofilms that contain less extracellular DNA remained intact. Standard plate counts of cells recovered from attached biofilms revealed a 4.1-log10 and a 3.9-log10 reduction in viable cells of wild-type PAO1 and pqsA mutant strains, respectively. Our results suggest that the action of photoactivated TMP on P. aeruginosa biofilms is two-fold: direct killing of individual cells within biofilms and detachment of the biofilm from the substratum. There was no evidence of porphyrin toxicity in the absence of light; however, biofilms pretreated with TMP without photoactivation were substantially more sensitive to tobramycin than untreated biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen known to cause infections in immunocompromised individuals and is the leading cause of mortality among cystic fibrosis (CF) patients [10]. The organism possesses a number of virulence factors that contribute to its ability to invade and colonize its host [19, 20]. In addition, it forms complex communities known as biofilms; hydrated matrices of cells consisting of polysaccharides, extracellular DNA, and proteins [5, 9, 13, 14, 23]. P. aeruginosa has been shown to form biofilms on abiotic (e.g., catheters and stents) as well as biotic (e.g., urinary tract and lung tissue) surfaces [2, 7, 18]. Biofilms are of significant medical importance because they confer the ability to evade the host immune system and render the cells more resistant to antimicrobial agents [3, 15]. These common characteristics lead to persistent and chronic infections [2].
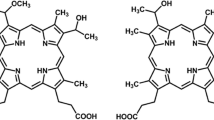
Photodynamic therapy (PDT) has been useful in the treatment of certain cancers and other diseases such as macular degeneration. In recent years, there has been increased interest in using PDT as a means to treat bacterial infections [22]. PDT requires three components: light, oxygen, and a photosensitizer. Light-activated cationic porphyrins transfer energy to molecular oxygen resulting in the production of singlet oxygen (1O2). This mechanism is known as the Type II reaction. 1O2 reacts with different components (e.g., phospholipids, peptides, and sterols) of the cell wall and cell membranes and also mediates DNA damage and cell death [21]. The cationic porphyrin 5,10,15,20-tetrakis(1-methyl-pyridino)-21H,23H-porphine, tetra-p-tosylate salt (TMP) specifically causes DNA damage by intercalating between DNA base pairs, causing photoinduced strand breakage when irradiated [12, 16].
Previous studies have demonstrated the ability of cationic porphyrins to successfully photoinactivate Gram-positive and Gram-negative bacteria, as well as fungi [11]. In this study, we examined the effectiveness of TMP against P. aeruginosa biofilms. TMP at a concentration of 2.5 mg ml−1 has been shown to reduce P. aeruginosa PAO1 planktonic cell populations by >102 cfu ml−1[8]. In the same study, it was demonstrated that higher concentrations (5.0 mg ml−1) of TMP were necessary to achieve the same level of killing in bacteria enmeshed within biofilms; however, they did not examine the effect of TMP on biofilm structure. Additionally, this porphyrin is known to significantly reduce S. aureus survival and, when combined with antibiotics, disrupt established biofilms [6]. In this study, we investigated the effects of photoactivated TMP on P. aeruginosa biofilms using two strains: a wild-type PAO1 strain and its isogenic pqsA mutant that has previously been shown to produce biofilms with highly reduced levels of extracellular DNA [1]. Additionally, we investigated the ability of TMP to affect biofilms in the absence of photoactivation.
Materials and Methods
Bacterial Strains, Growth Conditions, and Chemicals
The P. aeruginosa PAO1 wild-type and isogenic pqsA mutant strain were obtained from Eb Pesci (East Carolina University School of Medicine). P. aeruginosa strains were grown aerobically with shaking in Minimal Salts medium (40 mM K2HPO4, 20 mM KH2PO4, 7.6 mM [NH4]2SO4, 0.2 mM MgSO4·7H2O, 9.2 × 10−3 mM FeCl3·6H2O, 0.2% [wt/vol] glucose; adjusted pH 7.0) at 37°C [4, 17]. For static biofilms, P. aeruginosa strains were grown overnight in Minimal Salts medium at 37°C with shaking. The following day, bacteria were diluted in fresh media to an OD590nm of 0.15. Five hundred microliters of the standardized culture was added to sterile polystyrene cuvettes and incubated statically for 24 h at 37°C. For examination of static biofilms using confocal laser scanning microscopy (CLSM), sterile microscope slides were submerged in standardized cell suspensions and incubated statically at 37°C for 24 h.
Photosensitizer
5,10,15,20-tetrakis(1-methyl-pyridino)-21H,23H-porphine, tetra-p-tosylate salt (TMP) was purchased through Sigma-Aldrich. A 12.5 mg ml−1 TMP stock solution was prepared in dH2O and filter sterilized. TMP was added to cell suspensions and biofilms at various concentrations. TMP concentrations of 100 (0.14 mg ml−1) and 225 μM (0.35 mg ml−1) were chosen based on their effectiveness in preliminary trials.
Photoactivation
TMP was activated using a 100-Watt mercury vapor lamp fitted with a colored glass filter (Newport FSR-GG420) blocking wavelengths shorter than 400 nm. Samples were irradiated for various exposure times at an intensity of 220–240 Joules/cm2.
CLSM of Static Biofilms
Overnight biofilms formed on slides were rinsed in phosphate buffered saline (PBS), pH 7.0, and transferred to 50-ml tubes containing PBS supplemented with TMP at a concentration of either 100 or 225 μM. Negative control slides were transferred to 50-ml tubes containing only PBS. Following pre-exposure to TMP, biofilms were irradiated for 10 min and washed briefly in PBS. Bacterial viability was assessed in biofilm cultures using the LIVE/DEAD BacLight bacterial viability assay (Molecular Probes Inc., Eugene, OR), containing SYTO9 and propidium iodide dyes. Biofilms were visualized with an Olympus FV1000 CLSM (Olympus America, Center Valley, PA) using a 60× oil immersion objective. Biofilm images were acquired in 0.4-μm optical sections for the entire thickness of the biofilm.
Effect of TMP on Viability of Biofilm-Associated Cells
Static biofilms formed in sterile polystyrene cuvettes, as described above, were used to quantify cell survival. Supernatants from 24 h biofilms were removed and replaced with PBS containing TMP and irradiated for 10 min. Cells released from the biofilm following treatment were collected from the supernatant by centrifugation and resuspended in PBS. The remaining attached biofilm was washed once with PBS and attached cells were released from the surface using mechanical shearing by repeated pipetting. The number of viable cells present in the supernatants and biofilms following release by mechanical shearing were determined by plating on LB (1.5% agar) plates. Plates were incubated for 24 h at 37°C.
TMP Induced Degradation of DNA
pUC18 plasmid DNA (100 ng ml−1) was incubated with TMP at a final concentration of 100 or 225 μM. Plasmid DNA with and without TMP was irradiated for designated times. Prior to light exposure, samples of DNA supplemented with TMP were collected and incubated in the dark for comparison. DNA was examined using agarose gel electrophoresis.
Combined TMP and Tobramycin Treatment
Biofilms were grown on glass slides as described above. The 24 h biofilms were incubated for 10 min in 225 μM TMP, rinsed in PBS, and transferred to tubes containing MSG or MSG supplemented with 100 μg ml−1 of tobramycin. Slides were incubated in tobramycin for 2 h at 37°C. All incubations were performed in the dark. Cell viability within biofilms was assessed using the LIVE/DEAD BacLight bacterial viability kit and visualized with an Olympus FV1000 CLSM as described above.
Results
TMP Effects on Biofilm Structure and Cell Viability
The effect of TMP on 24 h P. aeruginosa PAO1 biofilms was assessed using CLSM and viable plate counts. In the absence of TMP, wild-type PAO1 cells formed dense biofilms on glass slides (Fig. 1a) and in polystyrene cuvettes (data not shown). When wild-type PAO1 biofilms were exposed to 100 μM TMP and irradiated for 10 min, there was a decrease in biofilm density and the majority of cells within the biofilm were nonviable based on LIVE/DEAD staining (Fig. 1c). Exposure to 225 μM TMP and 10 min of irradiation resulted in a nearly complete disruption and clearance of established wild-type PAO1 biofilms (Fig. 1e). The few remaining attached cells were nonviable. Shorter periods of light exposure or lower concentrations of TMP resulted in less clearance of the biofilms (data not shown). Interestingly, biofilms exposed to TMP but not irradiated appeared to be expanded in volume without a loss of cell viability (Fig. 1g).
Confocal laser scanning micrographs of P. aeruginosa biofilms. Biofilms were grown on glass slides for 24 h under static conditions in MSM and then exposed to specified concentrations of TMP or PBS as a negative control. Following exposure to TMP or PBS only, biofilms were either irradiated (a–f) with a 100-Watt mercury vapor lamp for 10 min or incubated in the dark (g–h). Bacterial viability was determined using the LIVE/DEAD BacLight Bacterial Viability assay. Cells staining red are considered dead while cells staining green are alive. The images show horizontal optical sections from the midpoint of the biofilms flanked by vertical optical sections in biofilms treated with: a, b PBS only, light; c, d 100 µM TMP, light; e, f 225 µM TMP, light; and g, h 225 µM TMP, dark
Standard plate counts of cells recovered from biofilms formed in polystyrene cuvettes were used to quantify the effects of photoactivated TMP. Wild-type biofilms exposed to 225 μM TMP and 10 min of irradiation exhibited a 4.1-log10 decrease in viable cells in the attached biofilm population (Fig. 2). There was a 4.5-log10 reduction in the number of viable cells in the recovered supernatants of wild-type PAO1 biofilms following the same TMP treatment (Fig. 2). The recovered supernatants contained the cells sloughed off as the result of TPM exposure and irradiation.
Effect of TMP and light irradiation on cell survival of P. aeruginosa biofilm-associated cells. Established biofilms of wild-type PAO1 and the pqsA mutant were treated with TMP and irradiated 10 min with a 100-Watt mercury vapor lamp. Cells were collected from the supernatant of treated biofilms, as well as from the remaining attached biofilm. Cell suspensions were diluted and plated onto LB (1.5% agar) plates and incubated 24 h at 37°C. CFU were used to determine the surviving fraction. Attached cells: no TMP  , 225 µM TMP
, 225 µM TMP  ; Supernatant cells: no TMP
; Supernatant cells: no TMP  , 225 µM TMP
, 225 µM TMP 
In contrast to wild-type biofilms, pqsA mutant biofilms were significantly different in overall structure. As previously noted, these biofilms are not confluent [1] (Fig. 1b). When exposed to 100 or 225 μM TMP and irradiated for 10 min there was a decrease in cell viability of attached cells (Fig. 1d and 1f). Standard plate counts of attached cells showed a 3.9-log10 decrease in cell viability at TMP concentrations of 225 μM (Fig. 2). Similarly, there was a 4.2-log10 reduction in cell viability of pqsA cells collected from supernatants of irradiated biofilms treated with 225 μM TMP (Fig. 2). Although exposure to TMP and irradiation resulted in cell death, this treatment did not lead to the disruption or clearance of the pqsA mutant biofilms observed with wild-type cells. Without photoactivation, TMP did not affect cell viability or disrupt the architecture of pqsA mutant biofilms (Fig. 1h).
DNA Degradation in the Presence of TMP
To determine the effect of TMP on DNA, pUCP18 plasmid DNA was exposed to TMP and irradiated. The untreated control samples had the three expected forms of plasmid DNA: covalently closed circles, relaxed circular, and linear cut (Fig. 3: lanes 1 and 7). Plasmid DNA exposed to light only appeared similar to the control (Fig. 3: lanes 2 and 8).
Gel electrophoresis analysis of plasmid (pUCP18) DNA treated with TMP and irradiated. Purified plasmid (pUCP18) DNA (100 ng ml−1) was exposed to either 0, 100, or 225 µM TMP and irradiated with a 100-Watt mercury vapor lamp for 0, 5, or 30 min. Top band: relaxed circle (nicked circle), Middle band: linear, Bottom band: covalently closed circles (supercoiled). Lane 1: 0 TMP, nonirradiated control DNA; Lane 2: 0 TMP, irradiated 5 min, Lane 3: 100 µM TMP, nonirradiated, Lane 4: 100 µM TMP, irradiated 5 min, Lane 5: 225 µM TMP, nonirradiated, Lane 6: 225 µM TMP, irradiated 5 min, Lane 7: 0 TMP, nonirradiated, Lane 8: 0 TMP, irradiated 30 min, Lane 9: 100 µM TMP, nonirradiated, Lane 10: 100 µM TMP, irradiated 30 min, Lane 11: 225 µM TMP, nonirradiated, and Lane 12: 225 µM TMP, irradiated 30 min
Plasmid DNA treated with 100 or 225 μM TMP without subsequent photoactivation resulted in retarded mobility of DNA (Fig. 3: lanes 3, 5, 9, and 11) as expected due to its ability to intercalate into DNA [12, 16]. The combination of TMP and irradiation for either 5 or 30 min resulted in the complete degradation of pUCP18 plasmid DNA at concentrations of 100 or 225 μM TMP (Fig. 3: lanes 4, 6, 10, and 12).
Effect of Tobramycin on TMP-Treated Biofilms
Wild-type PAO1 biofilms were exposed to TMP for 10 min followed by exposure to 100 μg ml−1 of tobramycin for 2 h with all steps performed in the dark. As noted above, TMP treatment without photoactivation resulted in an expansion and loss of biofilm density with no observable reduction in cell viability. In biofilms treated with tobramycin there was a reduction in cell viability which was limited to cells near the surface of the biofilms, where oxygen is most plentiful (Fig. 4a). Treatment with TMP and subsequent exposure to tobramycin resulted in substantial clearance of the biofilms and greater loss of cell viability throughout the biofilms than with either single treatment (Fig. 4b).
Confocal laser scanning micrographs of P. aeruginosa wild-type PAO1 biofilms treated with tobramycin only and TMP and tobramycin in the absence of light. Biofilms were grown on glass slides for 24 h at 37°C and then exposed to TMP (225 µM) for 10 min in the dark. Following exposure to TMP, biofilms were incubated with tobramycin (100 µg ml−1) for 2 h at 37°C. Bacterial viability was determined using the LIVE/DEAD BacLight Bacterial Viability assay. Cells staining red are considered dead while cells staining green are alive. The images show horizontal optical sections from the midpoint of the biofilms flanked by vertical optical sections in biofilms treated with: a tobramycin; and b TMP + tobramycin
Discussion
In this study, we examined the effects of the cationic porphyrin, TMP, on established P. aeruginosa biofilms. TMP exposure plus photoactivation resulted in a substantial reduction in the numbers of viable bacteria within established wild-type P. aeruginosa biofilms as shown by viability staining (Fig. 1c and e) and standard plate counts (Fig. 2). Bacterial killing required photoactivation, indicating that there was no dark toxicity associated with TMP (Fig. 1g).
Previous studies have demonstrated that TMP at higher concentrations (5.0 mg ml−1) than used in this study resulted in a 1.2-log10 reduction of wild-type PAO1 isolates grown in biofilms when irradiated for 5 min [8]. We were able to achieve higher rates of killing (4.1-log10 reduction) of biofilm-associated wild-type PAO1 cells using concentrations of TMP as low as 0.32 mg ml−1 (225 μM) (Fig. 2). This difference in killing rates can be attributed to the different conditions under which the established biofilms were grown and treated.
Previous studies did not evaluate the change in P. aeruginosa biofilm structure following treatment with TMP and light. In addition to killing biofilm-associated bacteria, treatment with TMP followed by irradiation resulted in substantial disruption and clearance of wild-type PAO1 biofilms (Fig. 1c and e). At a concentration of 225 μM TMP, wild-type biofilms were completely disrupted with few cells remaining attached (Fig. 1e). Without photoactivation, TMP did not lead to clearance of wild-type biofilms; however, there was a noticeable expansion in the biofilm and loss of density (Fig. 1g). These results indicate that TMP photoactivation affects biofilms in two ways: direct killing of cells and the disruption of the biofilm architecture. Additionally, while TMP toxicity is dependent on photoactivation, it is able to alter biofilm architecture in the absence of light by an unknown mechanism.
To determine if disruption of established P. aeruginosa wild-type biofilms was solely due to inactivation of biofilm-associated cells or also involved the extracellular matrix of the biofilm, we examined the effects of TMP on extracellular DNA. Extracellular DNA has previously been shown to be necessary for normal biofilm formation and contributes to the overall architecture [23]. We attempted to quantify extracellular DNA in biofilms following treatment with TMP and photoactivation compared with untreated biofilms using various DNA stains, such as Pico Green. We observed a decrease in biofilm DNA of samples treated with TMP and light but there was also a decrease in biofilm DNA of samples that were treated with TMP in the absence of light (data not shown). Because both TMP and Pico Green intercalate between base pairs, we were unable to determine if this decrease in fluorescence was proportional to a reduction in extracellular biofilm matrix DNA or due to competitive inhibition, i.e., the intercalation of TMP preventing binding of Pico Green.
Due to the difficulty in quantifying extracellular biofilm DNA following TMP exposure using established staining techniques, we assessed the effects of TMP on purified pUCP18 DNA and a pqsA mutant. Prior to irradiation, pUCP18 plasmid DNA mobility was retarded following exposure to TMP, indicating intercalation of TMP (Fig. 3, lanes 3, 5, 9, and 11). Exposure to TMP and subsequent photoactivation led to complete degradation of pUCP18 plasmid DNA (Fig. 3, lanes 4, 6, 10, and 12). These results coincide with previous studies that demonstrated that TMP intercalates between DNA base pairs, causing photocleavage of DNA [12, 16]. Thus, TMP is expected to intercalate into available extracellular DNA within biofilms and, upon irradiation, leads to disruption of the DNA. Degradation of extracellular DNA in the biofilm matrix using DNase has been previously shown to disrupt biofilm architecture and leads to the dissolution of the biofilm [23]. TMP photocleavage of DNA would similarly result in the disruption of biofilms.
The pqsA mutant, defective in a late portion of the P. aeruginosa quorum-sensing cascade, has been shown to produce biofilms with substantially lower levels of extracellular DNA [1]. In the presence of TMP and light, high levels of killing were observed in pqsA mutant biofilms (Fig. 1d and f). However, in contrast to wild-type biofilms, the biofilms formed by the pqsA mutant were not disrupted by this treatment. The inability of TMP photoactivation to disrupt pqsA biofilms could be attributed, in part, to the lack of extracellular DNA in these biofilms. We conclude that disruption of P. aeruginosa PAO1 wild-type biofilms by TMP and light treatment is partially due to its effect on the extracellular DNA matrix and not just photoinactivation of the cells within the matrix. However, we acknowledge that the lack of dissolution of pqsA mutant biofilms by TMP photoactivation may not be solely due to differences in DNA content as these biofilms differ from wild-type biofilms in a number of important ways.
The ability of TMP to intercalate into DNA, leading to an unwinding and expansion of the DNA volume, could explain the expansion of wild-type PAO1 biofilms treated with TMP but not exposed to light. This change in the architecture of P. aeruginosa biofilms treated with TMP in the absence of photoactivation led us to explore how this might affect the ability of antibiotics to kill bacteria within biofilms. We examined the combined effects of TMP and the antibiotic tobramycin in the dark on established biofilms. Treatment of wild-type PAO1 biofilms with tobramycin, the major front-line antibiotic used in the treatment of CF lung disease, did not result in substantial biofilm clearance and led to minimal killing of biofilm-associated cells (Fig. 4a). Killing was primarily localized to the top layer of the biofilm. In contrast, exposure of wild-type PAO1 biofilms to TMP prior to treatment with tobramycin resulted in significant biofilm clearance and enhanced killing of cells (Fig. 4b). One of the limitations of photodynamic therapy is the delivery of light to infections in deep tissue. These findings are especially important because they show that TMP can act to disrupt biofilm structure when activated by light and also by a light independent mechanism that enhances killing when combined with tobramycin. A light independent treatment has the potential to be applied when trying to eradicate P. aeruginosa biofilms that are not easily accessible to irradiation such as those associated with cystic fibrosis patients.
References
Allesen-Holm M, Barken KB, Yang L et al (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Costerton JW (2001) Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol 9:50–52
Craven RC, Montie TC (1983) Chemotaxis of Pseudomonas aeruginosa: Involvement of Methylation. J Bacteriol 154:780–786
D’Argenio DA, Calfee MW, Rainey PB et al (2002) Autolysis and autoagreggation of Pseudomonas aeruginosa colony morphology mutants. J Bacteriol 184:6481–6489
Di Poto A, Sbarra MS, Provenza G et al (2009) The effect of photodynamic treatment combined with antiobiotic action or host defence mechanism on Staphylococcus aureus biofilms. Biomaterials 30:3158–3166
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Donnelly RF, McCarron PA, Cassidy CM et al (2007) Delivery of photosensitisers and light through mucus: investigations into the potential use of photodynamic therapy for treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J Control Release 117:217–226
Freidman L, Kolter R (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690
Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574
Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease. Photochem Photobiol Sci 3:436–450
Kelly JM, Murphy MJ (1985) A comparative study of the interaction of 5,10,15,20-tetrakis(N-methylpyridinum-4-yl)porphyrin and its zinc complex with DNA using fluorescence spectroscopy and topoisomerisation. Nucleic Acids Res 13:167–184
Matsukawa M, Greenberg EP (2004) Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol 186:4449–4456
Nemoto K, Hirota K, Mukarami K et al (2003) Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy 49:121–125
Nickel JC, Ruseska I, Wright JB et al (1985) Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary tract catheter. Antimicrob Agents Chemother 27:619–624
Pasternack RF, Gibbs EJ (1996) Porphyrin and metalloporphyrin interactions with nucleic acids. Met Ions Biol Syst 33:367–397
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Speer AG, Cotton PB, Rode J et al (1988) Biliary stent blockage with bacterial biofilm. A light and electron microscopy study. Ann Intern Med 108:546–553
van Delden C (2004) Virulence factors in Pseudomonas aeruginosa. In: Ramos J-L (ed) Pseudomonas: virulence and gene regulation. Kluwer Academic/Plenum Publishers, New York, pp 3–45
van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Infect Dis 4:551–560
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28
Wainwright M (2009) Photoantimicrobials—So what’s stopping us? Photodiag Photodyn Ther 6:167–169
Whitchurch CB, Tolker-Nielsen T, Ragas PC et al (2002) Extracellular DNA is required for bacterial biofilm formation. Science 295:1487
Acknowledgments
We would like to thank Dr. Mark Masthay for his technical assistance in the determination of light intensity and Dr. W. Dietz Bauer for his input on the manuscript. This study was supported by “Merck Institute for Science Education.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Tracy L. Collins and Elizabeth A. Markus contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Collins, T.L., Markus, E.A., Hassett, D.J. et al. The Effect of a Cationic Porphyrin on Pseudomonas aeruginosa Biofilms. Curr Microbiol 61, 411–416 (2010). https://doi.org/10.1007/s00284-010-9629-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9629-y