Abstract
A non-culture approach was used to study the archaeal diversity in Lake Elmenteita, Kenya. Five different sampling points were selected randomly within the lake. Wet sediments and water samples were collected from each sampling point. In addition, dry mud cake was collected from three points where the lake had dried. DNA was extracted from these samples and the 16S rRNA genes were amplified using primers described to be Domain-specific for Archaea. Eleven clone libraries were constructed using PCR-amplified 16S rRNA genes. A total of 1,399 clones were picked and analysed via ARDRA. 170 ARDRA patterns were unique and the respective clones were selected for sequencing. 149 clones gave analysable sequences. BLAST analysis showed that 49 belong to the Domain Archaea while the others were either chimera or affiliated to eukaryotic taxa. Comparative sequence analysis of archaeal clones affiliated them to a wide range of genera. The order Halobacteriales was represented by members of the genera Natronococcus, Halovivax, Halobiforma, Halorubrum, and Halalkalicoccus. The highest percentage (46%) of the clones, however, belonged to uncultured members of the Domain Archaea in the order Halobacteriales. The results show that the archaeal diversity in the lake could be higher than previously reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soda lakes are characterised by high salinity and alkalinity and many represent highly productive environments [16]. Studies on the lakes of the East African Rift Valley in Kenya have shown that they are habitats for novel species of Bacteria and Archaea [9]. The ability to recover and analyse 16S rRNA genes directly from environmental DNA provides a means to investigate the taxonomic composition of microbial populations in any environment without the need for cultivation [1, 8, 15, 23, 36]. The application of this method to study marine bacteria and archaea has revealed large numbers of novel microorganisms, which appear to be largely unaffiliated with previous isolates from the same environment [7]. Such methods have also been applied to a number of soil, thermal, and hypersaline environments resulting in the description of as-yet uncultivated groups of both Bacteria and Archaea [1, 5, 17, 27, 28]. These novel phylotaxa expand our knowledge about diversity though they do not unravel their metabolic capabilities. Over the past years several archaeal species, all of which were relatively closely associated with the genera Natronobacterium and Natronococcus, have been identified in hypersaline soda lakes, i.e. Lake Natron, Little Lake Magadi and Lake Magadi [12, 34]. To date no detailed phylogenetic study had been done on Lake Elmenteita. The objective of this study was to apply a culture independent approach to elucidate the archaeal diversity in Lake Elmenteita.
Materials and Methods
Study Site
Lake Elmenteita is situated 0°27′ S, 36°15′ E on the floor of the Kenyan Rift Valley at 1776 m above sea level and has no direct outlet [18]. The region is characterised by a hot, dry and semi-arid climate with a mean annual rainfall of about 700 mm. Due to the high temperatures very high evaporation rates occur during the dry seasons leading to a reduction in the total surface area. The present size of Lake Elmenteita is roughly 20 km2 and the depths rarely exceed 1.0 m. According to Mwaura [21] the water temperature ranges between 30 and 40°C, the alkalinity of the water is high (1,200 mg CaCO3/l) and the pH is above 9 with a high concentration of carbonates, chlorides and sulphates.
Sampling Site Description and Sample Collection
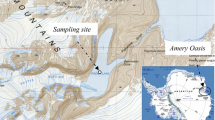
Water and mud (sediment) samples were collected from five different sampling sites (Fig. 1). At sampling site 1 the water was almost clear and warm, sampling site 2 harbours a hot spring (76°C) whereby water seeps from the rocks and flows into the lake. Turbidity increased from site 3–5 as a result of Cyanobacteria blooms and mixing by flamingos which feed on the cyanobacteria.
In addition three dry mud samples were collected from areas where the lake had dried out. Water samples were collected in sterile bottles, capped on site, labelled and preserved in cooled boxes for transportation back to the laboratory. Wet sediment and the dry mud samples were collected by scooping, placed into sterile 1.5 ml Eppendorf tubes, packed in sterile bags, labelled and preserved under dry ice. Wet sediment samples were labelled as ES1–ES5, water samples were labelled EW1–EW5 and the dry sediment samples were labelled ED3–ED5. The letter E refers to Elmenteita while S, W and D refer to Wet sediment, Water and Dry mud, respectively.
In the laboratory the water samples were initially filtered through Nucleopore filter (GF/F; Whatman) of 0.45 μm pore size and then through a glass fiber filter (Type GS; Millipore) of 0.22 μm pore size using a vacuum pump. The filter papers were properly folded in an aluminium foil, labelled and preserved in dry ice. The samples were shipped via courier service to DSMZ in Braunschweig, Germany, under dry ice for analysis. At the DSMZ, the samples were stored at −80°C.
DNA Extraction Protocol
The sediment and filter papers were thawn and 200 mg from each of the wet sediment sample weighed into a sterile 2 ml Eppendorf® tube. The filter sandwich was cut into small pieces with a sterile scalpel and transferred to a sterile 2 ml Eppendorf® tube. DNA extraction protocol was a modification of the method described by Sambrook and Russel [29]. In order to remove the salts and exopolysaccharides from the sediments samples, to each tube was added 500 μl of solution A (50 mM Tris pH 8.5, 50 mM EDTA pH 8.0, 25% sucrose solution) and mixed by gently inverting several times and centrifuged at 13,000 rpm for 1 min [4]. The supernatant was discarded and the sample re-suspended in 200 μl of solution A. 5 μl of Lysozyme (20 mg/ml), 5 μl of RNAse A (20 mg/ml) were added and mixed gently. The samples were allowed to incubate at 37°C for 1 h. To this mix was added 600 μl of solution B (10 mM Tris pH 8.5, 5 mM EDTA pH 8.0 and 1% SDS) and mixed by inverting several times after which 10 μl of Proteinase K (20 mg/ml) and mixed gently. The mix was incubated at 50°C for 50 min.
DNA extraction was via the method described by Sambrook and Russel [29]. Removal of humic substances from the DNA was done using the caesium chloride method [30]. Presence of DNA was checked on 1% agarose gel. The DNA was aliquoted in 10 μl. Short-term storage was at −20°C, while long storage was done at −80°C.
Almost full-length archaeal 16S rDNA genes were amplified using the primers arc8f (5′-TCCGGTTGATCCTGCC-3′) and arc1492r (5′-GGCTACCTTGTTACGACTT-3′) as described [31]. A gradient PCR was done a priori to check for the optimum annealing temperature. Thereafter PCR cycling consisted of a 3-min initial pre-incubation step at 94°C followed by 35 cycles of a denaturation step at 93°C for 1 min, a 1-min annealing step at 58°C, and a 1-min elongation step at 72°C and a final extension step at 72°C for 5 min. The PCR mix consisted of 5 ml of 10× PCR buffer [100 mM Tris–HCl (pH 9)], primers at a concentration of 0.5 mM, each deoxynucleoside triphosphate at a concentration of 200 mM, 2.0 mM MgCl2, 20 ng of bovine serum albumin (BSA), 0.5 μl of template DNA, 2.5 U of Taq DNA polymerase (Roche).The volume was adjusted to a final volume of 50 μl with sterile MQ water. The presence of PCR products and their concentration were determined by analysing 5 μl of product on 2% agarose gels.
Clone Library Construction
The PCR products were purified with QIAquick® spin columns (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The PCR products were eluted using 50 μl of TE Buffer (pH 8.0). 1 μl of the cleaned PCR products was ligated onto pGEM-T® Easy vector system II (Promega) and transfected through heat shock to E. coli JM109 high efficiency competent cells (Promega). Selection of transformants and extraction of plasmid DNA followed described protocols [3]. The presence of correct inserts was determined by performing a PCR using the primers M13F (5′- GTA AAACGACGGCCAG-3′) and M13R (5′-AGGAAACAGCTATGAC-3′). These primers flank the cloning site on the vector. The PCR products were checked on a 1% agarose gel. Further screening was done via ARDRA to select for a few representative clones for sequencing. Restriction digests of cloned PCR products were done using the restriction enzyme CviII from New England Biolabs [NEB] (Beverly, Mass.). Samples S4 and W5 were neither analysed by the ARDRA approach nor sequenced. Partial sequences were generated using the primer 514r. This primer is group specific and targets one of the conserved regions of the Domain Archaea [4]. Seven clones were sequenced using primer 1492r, as the forward primer resulted in unreadable sequences, probably due to the presence of microheterogeneities. The reads were manually edited and the sequence data were BLAST (www.ncbi.nlm.nih.gov/BLAST/) analysed against the GenBank 16S rRNA gene sequence database. The sequences were then aligned using the CLUSTAL W program against the nearest neighbours [14], and checked for chimeric structures by using the Mallard program [2]. Phylogenetic relationship of the partial sequences was determined using neighbour-joining [10] and maximum-likelihood analyses [22]. These analyses were conducted in MEGA 4 [32]. The evolutionary distances were computed using the Maximum Composite Likelihood method [27]. The resultant tree topologies were evaluated in bootstrap analyses of the Neighbour-joining method based on 1000 re-samplings [10]. Only representative partial sequences are indicated in the trees.
Nucleotide Sequence Accession Numbers
The sequences were deposited in GenBank under accession numbers FJ746846–FJ746893.
Results and Discussion
A total of 1,399 clones were picked and analysed via ARDRA. The selected restriction enzyme (CviII) does not cut the cloning vector. From the eleven samples, a total of 170 unique ARDRA patterns were selected for sequencing using the Primer 514r. Some patterns are indicated in Fig. 2.
Of the sequenced clones 149 gave good readable sequences. BLAST analysis, however, indicated that only 73 belonged to the domain Archaea, 24 of which were chimera, hence discarded, while the other 76 clones were affiliated to the 18S rRNA genes of eukaryotic groups such as bacterivorous nematode Diplolaimella dievengatensis (Clone EW4-023), Chlamydomonad spp (Clone EW4-011), Gelastocoris oculatus (Clone EW1-091), Ecumenicus monohystera (Clone ES3-039) and Brachionus plicatilis (Clone ES5-092). This indicates that the Archaeal primers were not domain-specific. A total of 49 non-chimeric archaeal sequences were aligned to the ARB database and all of them were affiliated to the phylum Euryarchaeota [11]. Of these, 13 were affiliated to cultured members while the remaining 36 were affiliated to as-yet-uncultured members of the phylum. Figure 3a shows the phylogenetic relationship of 38 clones to other members of the phylum Euryarchaeota.
a Evolutionary relationships of partial 16S rRNA gene clone sequences to selected taxa of the phylum Euryarchaeota, Domain Archaea, b Cluster 1 extrapolated from (a). Phylogenetic relationship of the partial sequences was determined using neighbour-joining [10] and maximum-likelihood analyses [22]. The analyses were conducted in MEGA 4 [33]. The evolutionary distances were computed using the Maximum Composite Likelihood method [32]. The resultant tree topologies were evaluated by bootstrap analyses based on 1000 re-samplings [10]. Only values above 70 are included
Sequence analysis indicates that the majority of the clones belonged to the family Halobacteriaceae. Members of this family require high salt for growth and are chemoorganotrophic, aerobic or facultatively anaerobic and are ubiquitous where salt concentration is high. BLAST analysis showed that seven clones belonged to the genus Natronococcus and the closest isolated relative was Natronococcus amylolyticus. Clone EW4-046 was 96% similar to Halobiforma lacisalsi but its position on the tree indicates that it could belong to the genus Natronococcus. Members of the Genus Natronococcus are alkaliphilic and require a pH of at least 8.5 for growth. Saline soda lakes are known to support blooms of halobacteria and harbour alkaliphilic representatives of the genera Natronobacterium and Natronococcus, Natronomonas, Natrialba, Natronorubrum and Halorubrum. Functionally, Halobacteria flourish on the organic matter concentration arising from evaporation of brine and the death of its microbial population [37]. Clones EW3-009, ED5-109 and EW3-004 formed a cluster and BLAST analysis showed they were affiliated to as-yet uncultured Halobacteriaceae. EW4-012 and clone ES2-019 were related to Halalkalicoccus tibetensis but with moderately high similarity values of 94 and 95%, respectively. Clones ES5-005 and ES5-058 were related to uncultured Halorubrum species whereas clone EW2-079 aligned with Halovivax asiaticus. Clone EW4-050 was 94% similar to Haloterrigena limicola
The remaining 20 halobacterial clones were affiliated to as-yet uncultured members of the family Halobacteriaceae. Within the 20 clones cluster 1 (Fig. 3b) of 15 highly similar sequences emerged, consisting exclusively of clones retrieved from Lake Elmenteita. Outside this cluster were the remaining five clones (ES1-010, ED3-042, ED5-024, EW1-001 and EW4-008) and these too were affiliated to as-yet uncultured Halobacteriaceae with a similarity value of 97–98%. Whether these clones originate from novel taxa have to await the characterisation of pure cultures. According to BLAST analysis, clone ES5-050 was affiliated to Methanocalculus. Members of the genus Methanocalculus obtain their metabolic energy via reduction of CO2 to methane whereas H2 and formate are electron donors. Their distinguishing feature is tolerance to high salt concentration.
Seven clones are not included in the tree since they could not be aligned with the other clone sequences as they were sequenced using the PCR primer 1492r. These seven clones showed BLAST similarity values below between 94 and 98% to both cultured and cultured members of the phylum Euryarchaeota. Clone EW1-009 was related to uncultured Methanospirillaceae whereas clones EW1-017 and EW1-087 were 98% similar to Methanocalculus pumilus. Within the order Methanomicrobiales, two clones (EW1-031 and EW4- 058) were affiliated to Methanosaeta, indicating that members of this taxon are represented in Lake Elmenteita. The remaining two clones (EW1-093 and EW1-025) were affiliated to uncultured euryarchaeota.
The findings of this study concur with other studies on other Kenyan soda lakes [4, 26], Lake Wadi-el-Natrun, Egypt [19] but also with the hypersaline, endoevaporitic microbial community in the pH-neutral brine of Eilat, Israel [25]. It is unclear whether the exclusive members of the phylum Euryarchaeota are due to primer bias or whether they are actually the only representatives of the archaeal domain within the soda lake environments. In a previous study by Rees et al. [26] on Lake Elmenteita, of the 14 archaeal-related amplicons retrieved, three were related to the genera Halobacteria, Haloarcula and Natronobacterium. DNA from the Halobacteriales has also been extracted from the Dead Sea, solar salterns, Antarctic hypersaline lakes, alkaline African hypersaline lakes, and Solar Lake, Sinai [5, 6, 20, 24]. Isolated strains of this group are aerobic halophiles growing at salinities up to NaCl precipitation, although some are capable of anaerobic growth either in the light using bacteriorhodopsin or in the dark by fermentation [13, 25].
A number of factors including relatively low cell numbers, a variable number of rRNA operons among organisms, as well as extraction and PCR bias, may lead to under-representation of phylotypes relative to their in situ abundance [35]. Therefore novel approaches to enrichment and isolation are needed to deepen our understanding of the roles played by the different groups within Lake Elmenteita.
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ (2006) New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl Environ Microbiol 72:5734–5741
Ausubel FM (1995) Current protocols in molecular biology. Wiley, New York
Baumgarten S (2003) Microbial diversity of soda lake habitats. Ph.D. thesis, Carolo-Wilhelmina University, Braunschweig
Benlloch S, Acinas SG, Anton J, Lopez-Lopez A, Luz SP, Rodriguez-Valera F (2001) Archaeal biodiversity in crystallizer ponds from a solar saltern: culture versus PCR. Microbiol Ecol 41:12–19
Cytryn E, Minz D, Oremland RS, Cohen Y (2000) Distribution and diversity of Archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl Environ Microbiol 66:3269–3276
De Long EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89:5685–5689
Dojka MA, Harris JK, Pace NR (2000) Expanding the known diversity and environmental distribution of an uncultured phylogenetic division of bacteria. Appl Environ Microbiol 66:1617–1621
Duckworth AW, Grant WD, Jones BE, van Steenbergen R (1996) Phylogenetic diversity of soda Lake Alkaliphiles. FEMS Microbiol Ecol 19:181–191
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Garrity GM, Holt JG (2001) Phylum AII. Euryarchaeota phy. nov. In Bergey’s manual of systematic bacteriology. In: Boone DR, Castenholz RW, Garrity GM (ed) The archaea and the deeply branching and phototrophic bacteria, 2nd edn, vol 1. Springer, New York, pp. 211–355
Grant S, Grant D, Brian EJ, Kato C, Li L (1999) Novel archaeal phylotypes from an East African alkaline saltern. Extremophiles 3:139–145
Hartmann R, Sickinger HD, Oesterhelt D (1980) Anaerobic growth of halobacteria. Proc Natl Acad Sci USA 77:3821–3825
Higgins DG, Sharp PM (1988) CLUSTAL:a package for performing multiple sequence alignments on a microcomputer. Gene 73:237–244
Hugenholtz P, Goebel B, Pace NR (1998) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. Int J Syst Bacteriol 180:4765–4774
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200
Ley RE, Harris JK, Wilcox J, Spear JR, Miller SR, Bebout BM, Maresca JA, Bryant DA, Sogin ML, Pace NR (2006) Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl Environ Microbiol 72:3685–3695
Melack JM (1988) Primary producer dynamics associated with evaporative concentration in a shallow, equatorial soda lake (Lake Elmenteita, Kenya). Hydrobiologia 158:1–14
Mesbah NM, Abou-El-Ela SH, Wiegel J (2007) Novel and unexpected prokaryotic diversity in water and sediments of the alkaline, hypersaline lakes of the Wadi An Natrun, Egypt. Microbiol Ecol 54:598–617
Moune′ S, Manach N, Hirschler A, Caumette P, Willison JC, Matheron R (1999) Haloanaerobacter salinarius sp. nov., a novel halophilic fermentative bacterium that reduces glycine-betaine to trimethylamine with hydrogen or serine as electron donors; emendation of the genus Haloanaerobacter. Int J Syst Bacteriol 49:103–112
Mwaura F (1999) A spatio-chemical survey of hydrogeothermal springs in Lake Elementaita. Kenya Int J Salt Lake Res 8:127–138
Olsen GJ, Woese CR (1993) Ribosomal RNA: a key to phylogeny. Fed Am Soc Exp Biol J 7:113–123
Olsen GJ, Lane DL, Giovannoni SJ, Pace NR (1986) Microbial ecology and evolution: a ribosomal RNA approach. Ann Rev Microbiol 40:337–365
Oren A (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol 28:55–63
Oren A, Trüper HG (1990) Anaerobic growth of halophilic archaeobacteria by reduction of dimethylsulfoxide and trimethylamine N-oxide. FEMS Microbiol Lett 70:33–36
Rees HC, Grant WD, Jones BE, Heaphy S (2004) Diversity of Kenyan soda Lake alkaliphiles assessed by molecular methods. Extremophiles 8:63–71
Robertson CE, Spear JR, Harris JK, Pace NR (2009) Diversity and stratification of archaea in a hypersaline microbial mat. Appl Environ Microbiol 75:1801–1810
Sahl JW, Pace NR, Spear JR (2008) Comparative molecular analysis of endoevaporitic microbial communities. Appl Environ Microbiol 74:6444–6446
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Smalla K, Cresswell N, Mendonca-Hagler LC, Wolters A, Van Elsas JD (1993) Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol 74:78–85
Sørensen KB, Canfield DE, Teske AP, Oren A (2005) Community composition of a hypersaline endoevaporitic microbial mat. Appl Environ Microbiol 70:7352–7365
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbour-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tindall BJ, Ross HNM, Grant WD (1984) Natronobacterium gen. nov. and Natronococcus gen. nov., two new genera of haloalkaliphilic archaebacteria. Syst Appl Microbiol 5:41–57
Von Wintzingerode F, Göbel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Ward DM, Weller R, Bateson MM (1990) 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature 345:63–65
Zavarzin GA, Zhilina TN, Kevbrin VV (1999) The alkaliphilic microbial community and its functional diversity. Microbiology (Moscow, English Translation) 68:503–521
Acknowledgements
This work was supported by DAAD within a Ph.D. scholarship (Sandwich model). The work was done at the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen), Braunschweig.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mwirichia, R., Cousin, S., Muigai, A.W. et al. Archaeal Diversity in the Haloalkaline Lake Elmenteita in Kenya. Curr Microbiol 60, 47–52 (2010). https://doi.org/10.1007/s00284-009-9500-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9500-1