Abstract
Pseudomonas sp. M18 is a rhizosphere isolate capable of producing two kinds of antifungal agents: phenazine-1-carboxylic acid (PCA) and pyoluteorin. Recently, the two well-studied quorum sensing (QS) systems of Pseudomonas aeruginosa, LasR/LasI and RhlR/RhlI, have also been identified in this strain. However, in this study, through the use of lacZ translational fusion expression analysis and acyl-homoserine lactone thin-layer chromatography (TLC) bioassays, we clearly display a more complex and distinctive hierarchy of the las and rhl QS systems in strain M18. In this QS cascade, expression of rhlI was negatively controlled by the LasR/LasI QS system. In contrast with lasI, which negatively regulated the rhlR induction, lasR exerted a positive influence on rhlR expression during the log-phase. This interrelationship indicated that the response regulators (LasR and RhlR) of the QS system are expressed independently of their cognate synthases (LasI and RhlI). Furthermore, the las system also modulated the timing and magnitude of the rhlI and rhlR maximal expression. In addition, our data imply that the lasR gene exerts its negative control on PCA production through modulation of rhlI expression. Thus, interactions between the two QS systems are strain specific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quorum sensing (QS) is a process employed by many bacteria for communication and for synchronization of group behavior. It is affected via the secretion of specific signal molecules (autoinducers) and occurs in a cell density-dependent manner. The QS system is typically comprised of the synthase gene, whose product catalyzes the biosynthesis of signals, and the receptor (regulator) gene, whose product responds to the signal and subsequently regulates the target genes.
The QS systems are widespread in pseudomonads. The most extensively studied of these are the las and rhl systems in strain Pseudomonas aeruginosa. The las QS system is composed of LasI, which directs the synthesis of the signal molecule N-3-oxododecanoyl-homoserine lactone (OdDHL, also known as 3-oxo-C12-HSL) and of its receptor LasR. Similarly, the rhl QS system consists of RhlI, which is responsible for the synthesis of the signals N-butanoyl-homoserine lactone (BHL, major product, also known as C4-HSL) and N-hexanoyl-homoserine lactone (HHL) and of its receptor RhlR. The two systems themselves are subject to a positive feedback and regulate overlapping sets of genes concerning the virulence factor production, biofilm formation, and many other genes [1]. Some of these, such as rhlAB (rhamnolipid) and the phzABCDEFG operon (phenazine biosynthesis), have been identified as being specifically and directly activated by the rhl regulon. For other numerous las-controlled genes, the situation is not as clear [2–4]. Previously published studies have shown that the two systems are organized hierarchically and are interdependent, so that the las QS system has a positive effect on rhlI and rhlR expression [5, 6].
Pseudomonas sp. M18 is a fluorescent Pseudomonas strain that can suppress diseases caused by pathogenic fungi in crop plants. This biocontrol capacity largely depends on the production of its two kinds of antibiotics: phenazine-1-carboxylic acid (PCA) and pyoluteorin (Plt). Strain M18 has a similar genetic background to that of P. aeruginosa PAO1, shown by the 99% identity between the 16S rRNA gene sequences of M18 (AY696302) and PAO1. Although the production of antifungal compounds (e.g., phenazine derivatives) is common in the strains of P. aeruginosa, some marked characteristics of strain M18 clearly reflect that it is distinct from P. aeruginosa strain reported before, such as Plt production and its biosynthetic structural, regulatory, and ABC export (or resistance) gene cluster (GenBank accession number AY394844), the high production of PCA, and remarkably different regulatory mechanisms of secondary metabolisms including Plt and PCA biosynthesis [7–10].
Recently, two complete las and rhl QS systems identified in strain M18 have been shown to play critical roles in the regulation of secondary metabolites [7, 10]. However, the las QS system in strain M18 has been reported to negatively control phzABCDEFG expression. In contrast, the las system in P. aeruginosa mutants exhibits a significant delay of phzABCDEFG expression and pyocyanin production, through modulation of the rhl regulon [7, 11]. The aim of this study was therefore to further investigate this apparent discrepancy, explore the role of the rhl system on PCA production, and the interaction between the las and rhl systems in Pseudomonas sp. M18.
Materials and Methods
Bacterial Strains and Growth Conditions
Pseudomonas sp. M18 is a fluorescent Pseudomonas strain isolated from the watermelon rhizosphere. It is an unusual strain in that it shares some distinct features with both P. aeruginosa and Pseudomonas fluorescens [7–10]. The other bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was routinely grown at 37°C in Luria-Bertani (LB) medium. Pseudomonas sp. M18 and its derivatives were incubated at 28°C in King’s medium B (KMB) [12] or pigment-producing medium (PPM) [13]. The antibiotics added to media were ampicillin (Ap) (100 μg ml−1), spectomycin (Sp) (100 μg ml−1), kanamycin (Km) (50 μg ml−1), tetracycline (Tc) (120 μg ml−1), and gentamicin (Gm) (40 μg ml−1) for pseudomonads, and Ap (100 μg ml−1), Km (50 μg ml−1), Tc (15 μg ml−1), and Gm (10 μg ml−1) for E. coli.
Construction of lasR rhlI Double Genes Mutant from rhlI Mutant Strain M18IG
The plasmid pMETcLRK was first transformed into the E. coli SM10. Then it was mobilized from the E. coli SM10 donor into strain M18IG by biparental mating. Transconjugants were selected on LB plates containing Sp to counter-select E. coli SM10 and Km. After a second crossing-over, Km-resistant, Tc-sensitive, and sucrose-resistant recombinants with the chromosomally inactivated lasR gene in strain M18IG were obtained. The resultant lasR rhlI double mutant, designated M18IR, was confirmed by PCR and sequencing with the primers LasR-UP and LasR-Down [7].
Construction of Translational rhlR′–′lacZ Fusions
A 357-bp PCR fragment carrying the promoter region and part of the rhlR gene was amplified from the strain M18 genomic DNA using primers PrhlR-UP (5′-CGT CGA ATT CTG TCA CAA CCG CAC AGT ATC-3′) and PrhlR-DOWN (5′-ACT GCT GCA GAT AGG CGT AGT AAT CGA AGC-3′) (EcoRI and PstI sites are underlined). The 357 bp PCR fragment shared 99.5% identity with that from P. aeruginosa PAO1. The fragment was cloned into EcoRI-PstI-digested pME6015 to generate pRRL, a translational rhlR′–′lacZ fusion.
Quantitative Assay of PCA by HPLC
Pseudomonas sp. M18 and its derivative strains were grown in PPM media as indicated in the caption of Fig. 1. Extraction and quantification of PCA were carried out as previously described [7].
β-Galactosidase Assay
Pseudomonas sp. M18 and its derivatives containing the lacZ reporter plasmids were cultivated in KMB media, as indicated in the caption of Fig. 3. The β-galactosidase activities were assayed as previously described [7].
TLC Analysis of Acyl-Homoserine Lactone (AHL) Signals
The AHL signals, BHL and HHL, were detected using a TLC bioassay, as described by McClean et al. [14] and Shaw et al. [15]. M18 and its derivative strains were grown to an OD600 of 7.5 to 8.5 in KMB media. The AHL extraction and analysis were performed as described previously [10]. Synthetic AHL standards of BHL and HHL (catalogue no. 09945 and 09926; Fluka, Shanghai, China) served as controls. Chromobacterium violaceum CV026 was grown overnight at 28°C in LB broth and used as the indicator strain [14, 15].
Results
The Effect of QS System on PCA Production
The las and rhl systems in strain M18 appeared to be organized in a different manner to that seen in P. aeruginosa, in terms of the production of PCA. As shown in Fig. 1, lasR disruption led to a substantial enhancement of PCA production, in agreement with our previous report that the las system negatively controls PCA biosynthesis in strain M18 [7]. A similar reduction in PCA production was caused by the rhlI mutation and the lasR rhlI double mutation (Fig. 1). This result not only indicates that rhlI positively controls PCA biosynthesis, but also implies that lasR may exert some effect through rhlI.
The Regulation of las QS System on the rhlI′–′lacZ Translational Fusion Expression
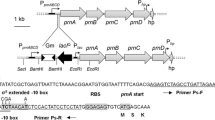
To confirm the latter hypothesis and to determine the influence of las QS system on the rhl QS system, we performed lacZ-based fusion analysis in strain M18 and its lasI or lasR inactivated mutant. The regulation of las and rhl QS systems may occur at transcriptional and/or post-transcriptional levels in strain M18. Since the rhlI′–′lacZ and rhlR′–′lacZ transcriptional fusions cannot reflect post-transcriptional regulation of rhlI and rhlR, we constructed two translational fusions pMEIZ (rhlI′–′lacZ) and pRRL (rhlR′–′lacZ) to, respectively, assay the rhlI and rhlR expressions. These two translational fusions contain the flanking region of the transcriptional start site (TSS) and the first several codons of rhlI and rhlR, they can, respectively, reflect the combined transcriptional and translational expression levels of rhlI and rhlR (Fig. 2c). The rhlI and rhlR genes expression in corresponding strains were monitored in KMB media throughout cell growth, as described in Fig. 2. Both rhlI and rhlR genes were expressed throughout the growth cycle in the wild-type strain M18. During the early exponential phase of growth both lacZ fusions were expressed at a relatively low level, but they were induced rapidly and reached maximal expression during the late exponential phase. This observation was in accordance with that seen in P. aeruginosa [5, 16]. In contrast, as reflected in Fig. 2a, both lasI and lasR mutants followed a similar pattern of rhlI′–′lacZ expression as that seen in the parent strain M18. However, the rhlI′–′lacZ expression in the strains showing disrupted las QS systems was significantly enhanced during some growth phases as compared with that seen in the wild-type strain. This increase could be readily observed at the maximal expression level, where the rhlI′–′lacZ expression was about twofold higher in the lasI or lasR mutants than it was in strain M18. Taken together, the las QS system appeared to be negatively controlling the rhlI expression in strain M18 (Fig. 2a). Furthermore, the maximal expression of rhlI′–′lacZ fusion in the las QS mutants was restricted to the early stationary phase, while it occurred at the late log-phase growth in the wild-type strain.
Effect of LasI and LasR on rhlI and rhlR gene expression levels and cell growth. OD600 (open symbols) value and β-galactosidase expression (solid symbols) of the rhlI′–′lacZ translational fusion expression plasmid pMEIZ (a), the rhlR′–′lacZ translational fusion expression plasmid pRRL (b) were followed over time in cultures of wild type M18 (square), lasI mutant M18LIG (circle), and lasR mutant M18LRK (triangle) in KMB media. Data represent the means (±SD) of triplicate cultures. (c) Maps of plasmid pRRL and pMEIZ. S/D, putative Shine-Dalgarno sequence. +1, putative TSS. The putative promoter region and its flanking sequence are shown as a thick black line
The Influences of the las QS System on rhlR′–′lacZ Expression
As shown in Fig. 2b, the delayed maximal expression of the rhlR′–′lacZ reporter was also observed in both lasI and lasR mutant strains. However, the rhlR′–′lacZ fusion exhibited significantly different patterns of expression in the lasI compared to the lasR mutants. Both basal and maximal levels of the rhlR gene expression in the lasI mutant were increased by approximately twofold, compared with those of the parental strain, although both strains had a similar expression profile (Fig. 2b). In contrast, the activity of rhlR′–′lacZ fusion in the lasR mutant was greatly reduced overall during the exponential phase, compared with the parental M18 strain, although it still showed a linear increase. Upon entry into the early stationary phase, the rhlR expression in the lasR mutant was substantially activated and showed maximal rhlR expression about twofold higher than that in the parental M18 strain (Fig. 2b). Overall, the lasI gene appeared to exert a negative control over rhlR expression in strain M18. However, the basal level of rhlR expression was positively regulated by lasR during the log-phase and the higher maximal activities of rhlR expression were delayed until the early stationary phase in lasR mutant.
The Modulation of QS Systems on BHL and HHL Production
TLC bioassay has been reported to detect and semi-quantify the AHLs signal molecules. The intensity of the indicator strain’s response to signal molecules can reflect the levels of AHLs amounts [14, 15]. To further corroborate the hypothesis that the rhlI gene was negatively modulated by the las QS system, TLC assay was performed to evaluate the levels of the signal molecules BHL and HHL in the wild-type strain M18 and its derivative strains when grown to an OD600 of 7.5 to 8.5. As shown in Fig. 3, neither BHL nor HHL was detected in the rhlI mutant strain, in agreement with the supposition that RhlI is responsible for the synthesis of these two effectors [10]. The chromosomal inactivation of lasI or lasR resulted in an increase in BHL and HHL production compared to that in the parental M18 strain. Conversely, the lasI or lasR overexpression (LIO and LRO in Fig. 3) led to a reduction in both BHL and HHL production in comparison with that observed in corresponding control strains (LI6 and LR6 in Fig. 3), which carry the empty vector pME6032 excluding the influence of plasmid on the BHL and HHL production (Fig. 3). The semi-quantified result of TLC bioassay is in consistent with the hypothesis above that the las QS system has a negative effect on the rhlI expression. In addition, the rhlR mutant strain produced similar amounts of BHL and HHL as did the wild-type strain (Fig. 3), indicating that the rhlI expression may be independent of the rhlR gene in strain M18.
TLC analysis of AHLs secreted by the wild-type M18 strain and its derivatives grown in KMB media. AHL samples were visualized with the C. violaceum CV026 reporter strain. M BHL and HHL markers, W the wild-type M18 strain, RI the rhlI mutant M18IG strain, RR the rhlR mutant M18RK strain, LI the lasI mutant M18LIG strain, LR the lasR mutant M18LRK strain, LI6 the M18LIG strain harboring the empty vector pME6032, LR6 the M18LRK strain harboring the empty vector pME6032, LIO the M18LIG strain harboring pME6032lasI in which the lasI gene was overexpressed, LRO the M18LRK strain harboring pME6032lasR in which the lasR gene was overexpressed
Discussion
In this study, rhlI specifically upregulated PCA production, which is in accordance with similar observations in P. aeruginosa. In addition, lasR exerted a negative control over phenazine biosynthesis through the intermediate modulation of rhlI. Thus, in contrast to the situation in P. aeruginosa, lasR may have a negative effect on rhlI expression.
Pseudomonas sp. M18 appeared to employ a novel and complex hierarchical cascade of las and rhl systems that was complex and distinctly different from that seen in P. aeruginosa (Fig. 4). The rhlI gene expression was negatively controlled by the las system. This conclusion can be drawn from the lacZ-based fusion analysis (Fig. 2b) and TLC bioassay (Fig. 3). As shown in Fig. 2b, lasI negatively regulated the expression of rhlR, while the inactivation of lasR gene resulted in a significant delay and overexpression of rhlR activities. This might imply the LasR acts primarily as an activator of the rhlR expression that predominates during the exponential growth phase. A surrogate activator of lasR or another inducer of maximal activation may exist during the stationary phase. In previous studies of P. aeruginosa, control of rhl expression was apparently achieved by more than just the las QS system [5, 11].
The expression of the QS regulatory gene was not fully coupled with its cognate synthase gene. As indicated in Fig. 2, the rhlR-fusion expression was differentially modulated by lasI and lasR. Likewise, rhlI and rhlR were also regulated by lasR in distinctly different ways in strain M18. These findings were inconsistent with those reported for P. aeruginosa where both LasI and LasR have a positive effect on the expression of rhlI and rhlR [5, 6]. This is explainable since the QS regulatory genes (lasR and rhlR) and their cognate synthase genes (lasI and rhlI) have been shown to be transcriptionally coupled and they function synergistically in P. aeruginosa [1]. This significant difference between strains M18 and P. aeruginosa provides an obvious hint that the major regulators (lasR and rhlR) of QS system are expressed and may work independently of their cognate synthase genes (lasI and rhlI) in strain M18. As shown in Fig. 3, the rhlR mutation had little effect on either BHL or HHL production, demonstrating that rhlI could be expressed independent of rhlR.
In P. aeruginosa, similar observations were reported, although it has been shown that the las and rhl systems are subject to a self-reinforcing positive feedback [1]. A recent report has suggested that the correlation between each synthase and its cognate transcriptional regulator can vary under different growth conditions [16]. More recently, a lasR (or rhlR) mutant was shown to retain its capacity to express lasI (or rhlI) and to produce its corresponding signal 3-oxo-C12-HSL (or C4-HSL) [7, 11, 17]. Furthermore, there is increasing evidence that implicates the QS signal molecule (3-oxo-C12-HSL or C4-HSL), or its receptor (LasR or RhlR) alone, in a function for avoiding the wasting of resources. Thus, the signal molecules have been shown to work independently of their corresponding regulators (LasR and RhlR) in many circumstances, including in vivo inflammatory responses and intra- and inter-species communication [18–22]. In addition, RhlR can regulate rhlAB expression, with or without the autoinducer [23]. Both of these molecules can bind to QscR and regulate the expression of other genes [24–26].
In strain M18, QS regulation does not appear to be a straightforward process. The expression of a receptor and its cognate synthase were not correlated with each other and this feature may allow fine-tuning of each regulator. Further insights into the nature of the interrelationship between the las and rhl systems in M18 will require detailed investigation into the regulatory mechanism of each of its major QS components.
The las QS system affected the timing and strength of rhlI and rhlR maximal expression. As shown in Fig. 2, the chromosomal inactivation of either the lasI or lasR gene caused a lag in the maximal expression of rhlI and rhlR fusion during the early stationary phase. A similar delayed effect resulting from las QS system disruption has also been reported in P. aeruginosa [11]. However, unlike P. aeruginosa, the las QS mutants of M18 expressed a much higher maximal activity of both the rhlI and rhlR genes than did their parental strain. This type of effect of the lasR mutation is conceivable since the las QS system primarily functions to activate gene expression in response to cell density.
Finally, it is also worth noting that the same experimental effects were observed with mutants grown in standard conditions in LB medium (data not shown). However, the las and rhl QS systems are known to be directly or indirectly regulated by a number of transcriptional regulators, and consequently, their expression is sensitive to environmental conditions. In P. aeruginosa PAO1, it has been reported that the relative timing and strength of expression of these two systems varied significantly under different conditions [16]. Given both the KMB and LB mediums utilized in this study are rich mediums, more systematical experiments under different conditions should be designed to further investigate the influence of environmental factors on lasR/I and rhlR/I genes expression and their regulatory interrelationship.
In conclusion, complicated interaction between the las and rhl QS systems occurs in Pseudomonas sp. M18 that is significantly different from the QS hierarchy reported in P. aeruginosa. These differences occur even though both organisms share many similar genetic features indicating the strain specificity of QS cascade. Genome sequencing of strain M18 is being undertaken and it will better clarify the genetic basis and classification of the strain. Additionally, since TLC bioassay is a semi-quantified method and influenced by many factors, to more accurately evaluate the amounts of AHLs and subtly study the regulation of AHLs productions, HPLC assay of AHLs is needed to be established. Our previous work has revealed that the las and rhl QS systems control expression of many genes in strain M18, more complete understanding of the las and rhl QS hierarchy will aid in the elucidation of complex connected regulatory network and the development of engineered strains with high production of PCA or Plt. At the same time, it will also help to decipher the evolution and regulatory mechanism of the QS system in Pseudomonas as compared with that in other pseudomonads.
References
Juhas M, Eberl L, Tummler B (2005) Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol 7:459–471
Latifi A, Winson MK, Foglino M, Bycroft BW, Stewart GS, Lazdunski A, Williams P (1995) Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol 17:333–343
Schuster M, Greenberg EP (2007) Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics 8:287
Whiteley M, Lee KM, Greenberg EP (1999) Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:13904–13909
Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132
Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A (1996) A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol 21:1137–1146
Chen Y, Wang X, Huang X, Zhang X, Xu Y (2008) Las-like quorum-sensing system negatively regulates both pyoluteorin and phenazine-1-carboxylic acid production in Pseudomonas sp. M18. Sci China C Life Sci 51:174–181
Huang X, Yan A, Zhang X, Xu Y (2006) Identification and characterization of a putative ABC transporter PltHIJKN required for pyoluteorin production in Pseudomonas sp. M18. Gene 376:68–78
Huang X, Zhu D, Ge Y, Hu H, Zhang X, Xu Y (2004) Identification and characterization of pltZ, a gene involved in the repression of pyoluteorin biosynthesis in Pseudomonas sp. M18. FEMS Microbiol Lett 232:197–202
Yan A, Huang X, Liu H, Dong D, Zhang D, Zhang X, Xu Y (2007) An rhl-like quorum-sensing system negatively regulates pyoluteorin production in Pseudomonas sp. M18. Microbiology 153:16–28
Dekimpe V, Deziel E (2009) Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155:712–723
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Levitch ME, Stadtman ER (1964) A study of the biosynthesis of phenazine-1-carboxylic acid. Arch Biochem Biophys 106:194–199
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711
Shaw PD, Ping G, Daly SL, Cha C, Cronan JE Jr, Rinehart KL, Farrand SK (1997) Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci U S A 94:6036–6041
Duan K, Surette MG (2007) Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J Bacteriol 189:4827–4836
Sandoz KM, Mitzimberg SM, Schuster M (2007) Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A 104:15876–15881
Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD (2005) Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci U S A 102:309–314
Qazi S, Middleton B, Muharram SH, Cockayne A, Hill P, O’Shea P, Chhabra SR, Camara M, Williams P (2006) N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun 74:910–919
Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-Zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP (2006) Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol 8:1601–1610
Zhu H, Conibear TC, Thuruthyil SJ, Willcox MD (2008) Pseudomonas aeruginosa quorum-sensing signal molecules induce IL-8 production by human corneal epithelial cells. Eye Contact Lens 34:179–181
Williams P (2007) Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153:3923–3938
Medina G, Juarez K, Valderrama B, Soberon-Chavez G (2003) Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J Bacteriol 185:5976–5983
Ledgham F, Ventre I, Soscia C, Foglino M, Sturgis JN, Lazdunski A (2003) Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol Microbiol 48:199–210
Lee JH, Lequette Y, Greenberg EP (2006) Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol Microbiol 59:602–609
Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP (2006) A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J Bacteriol 188:3365–3370
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Heeb S, Blumer C, Haas D (2002) Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J Bacteriol 184:1046–1056
Huang X, Zhang X, Xu Y (2008) Positive regulation of pyoluteorin biosynthesis in Pseudomonas sp. M18 by quorum-sensing regulator VqsR. J Microbiol Biotechnol 18:828–836
Acknowledgments
This study was supported by the Hi-Tech Research and Development Program (863 Program) of China (No. 2007AA02Z215), the National Natural Science Foundation of China (No. 30800009), the National Key Basic Research Program (973 Program) of China (No. 2009CB118906), and the Key Project of the Shanghai Committee of Science and Technology (No. 08391911900). We are very grateful to the referees and editor for their helpful and valuable suggestions and revisions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lu, J., Huang, X., Zhang, M. et al. The Distinct Quorum Sensing Hierarchy of las and rhl in Pseudomonas sp. M18. Curr Microbiol 59, 621–627 (2009). https://doi.org/10.1007/s00284-009-9483-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9483-y