Abstract
Characterization of bacteriophages to be used prophylactically or therapeutically is mandatory, as use of uncharacterized bacteriophages is considered as one of the major reasons of failure of phage therapy in preantibiotic era. In the present study, one lytic bacteriophage, KPO1K2, specific for Klebsiella pneumoniae B5055, with broad host range was selected for characterization. As shown by TEM, morphologically KPO1K2 possessed icosahedral head with pentagonal nature with apex to apex head diameter of about 39 nm. Presence of short noncontractile tail (10 nm) suggested its inclusion into family Podoviridae with a designation of T7-like lytic bacteriophage. The phage growth cycle with a latent period of 15 min and a burst size of approximately 140 plaque forming units per infected cell as well as a genome of 42 kbps and structural protein pattern of this bacteriophage further confirmed its T7-like characteristics. Phage was stable over a wide pH range of 4–11 and demonstrated maximum activity at 37°C. After injection into mice, at 6 h, a high phage titer was seen in blood as well as in kidney and urinary bladder, though titers in kidney and urinary bladder were higher as compared to blood. Phage got cleared completely in 36 h from blood while from kidneys and urinary bladder its clearance was delayed. We propose the use of this characterized phage, KPO1K2, as a prophylactic/therapeutic agent especially for the treatment of catheter associated UTI caused by Klebsiella pneumoniae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriophages are viruses that infect bacteria and consist of DNA or RNA enclosed within a protein coat. Recently bacteriophages have been used extensively for various purposes but the use of bacteriophages for therapeutic purposes has come a long way since its first use by d’Herelle in 1920s. Several studies show that bacteriophages could be used successfully for therapeutic purposes both in humans and animals [7, 10, 20, 21, 30]. Phages not only have been used in therapy but also as immunomodulatory agents to help our immune system eliminate endogenous and exogenous pathogens [16] besides inhibiting production of reactive oxygen species in response to bacteria and endotoxins [24]. However, despite the efficacy of bacteriophage in biological applications there are concerns about the use of bacteriophages [32].
It has been postulated that about 1030 bacteriophages are present in the environment [4, 17, 29]. Despite this rich resource of bacteriophages present in the environment only about 300 phages have been characterized [8]. One of the major reasons for failure of phage therapy in preantibiotic era was the application of uncharacterized bacteriophage preparations that led to inconsistent outcomes of the therapy. Hence isolation and characterization of new phages is very important especially in the light of observation that most of disease causing organisms live in matrix enclosed environments called biofilms [31] that inherently show resistance toward antibiotics [14]. Even though, for biofilm eradication phages can be used alone as biofilms offer little or no resistance to bacteriophages and their encoded enzymes [28] but certain recent studies [6, 11] reporting the synergistic application of phages and antibiotics have further broaden the horizon for bacteriophage application. But for all these purposes characterized phages are required. Hence, in the present study an attempt has been made to characterize a bacteriophage for its in vitro as well as in vivo characteristics to assess its potential for therapy.
Materials and Methods
Bacterial Strains and Culture Conditions
Bacterial strains used in the present study have been enlisted in the Table 1. All bacterial cultures were grown aerobically in nutrient broth. Stocks of bacterial cultures were made in nutrient broth with added glycerol (60%) and stored at −80°C. Prior to phage sensitivity bacteria were subcultured at least twice in tryptone soya broth. For all phage experiments 4–6 h bacterial growth, unless otherwise stated, was used.
Bacteriophage Isolation, Detection, and High Titer Preparation
Klebsiella pneumoniae B5055 specific bacteriophages were isolated from sewage water by enrichment technique. Water sample was centrifuged (10,000 rpm/15 min) and to the clear supernatant 5% (v/v) host bacterial culture (overnight growth) along with equal volume of nutrient broth was added and incubated at 37°C overnight. Next day after centrifugation (10,000 rpm/20 min) phage activity in the supernatant was detected by spot assay [9]. Titer of the phage preparation was estimated by agar overlay method [3] and titer was expressed in plaque forming units (pfu)/ml. To ensure a pure phage preparation several plaque purification steps were performed wherein a single isolated plaque was cut using a sterile spatula and inoculated into fresh 6 h growth of K. pneumoniae and incubated (37°C). Ultimately isolated phage was ascertained of its purity by homogeneity of plaque morphology. Isolated phage was named KPO1K2, with the first two letters indicating the host genus and the species name [2]. It was further concentrated by polyethylene glycol (PEG) precipitation [33].
Single-Step Growth Experiment
One step growth curve of KPO1K2 was performed according to the method of Pajunen et al. [25] as modified by Sillankorva et al. [27]. Optical density (OD) of mid-exponential bacterial culture at 600 nm was adjusted to a corresponding cell density of 108 cfu/ml. To 5 ml of this bacterial culture was added 5 ml of bacteriophage suspension in order to have a multiplicity of infection (MoI) of 0.001. Phages were allowed to adsorb for 5 min at room temperature. The mixture was then centrifuged (10,000 rpm/5 min) and pellet was resuspended in 1.0 ml of nutrient broth. Samples (100 μl) in duplicate were taken at 5 min interval and subjected to phage titration. First set of sample was immediately diluted and plated for phage titration while other set was treated with 1% (v/v) chloroform to release intracellular phages in order to determine the eclipse period. Experiment was performed at three different occasions and values depict the mean of three observations ± standard deviation (SD).
Electron Microscopy
Bacteriophage sample was concentrated by ultra centrifugation at 100,000 rpm/2 h (Sorvall M150 GX, Newton, CT, USA). Concentrated viral samples were adsorbed onto carbon coated copper grids and negatively stained with 4% phosphotungstic acid (pH 4.0), and viewed under an electron microscope (EM; Hitachi H 7500, Tokyo, Japan) at 80 kV at Sophisticated Analytic Instrumentation Facility (SAIF), Panjab University, Chandigarh, India. The phage size was determined from the average of 5–7 independent measurements.
DNA Isolation and Restriction Endonuclease Analysis
Bacteriophage DNA was extracted and purified from phage lysates by proteinase K method, followed by resuspension in Tris–EDTA buffer after ethanol precipitation as described earlier [23]. DNA from phage was digested with the restriction endonucleases EcoRI and Sau3A1 (Bangalore Genie, Bangalore, India), BsuRI and HinfI (MBI Fermentas), according to the suppliers’ recommendations. DNA fragments were separated by electrophoresis in 0.8% agarose gel containing ethidium bromide (0.5 μg/ml) in 1× Tris–acetate–EDTA buffer, at 80–100 V in a Bio-Rad agarose gel electrophoresis system (Bio-Rad Laboratories). Fragment sizes were determined from HindIII digests of lambda DNA and 1 kb DNA ladder (Bangalore Genie, Bangalore, India). Restriction digestion was repeated at least thrice to ascertain the results.
Bacteriophage Structural Protein Analysis by SDS-PAGE
Structural proteins of PEG precipitated KPO1K2 were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) [5]. Phage particles purified partially by PEG precipitation [21] were mixed with the sample buffer and heated in a boiling water bath for 3 min, followed by separation of proteins in the gel (10%). Protein bands were visualized by staining the gel with Coomassie brilliant blue.
Host Range Determination
The strains to be tested were grown overnight in nutrient broth. Three milliliters of the molten soft nutrient agar (0.7%) was mixed with 100 μl of bacterial cells, which was then overlaid on the surface of solidified basal nutrient agar (1.5%). A total of 10 μl (ca. 1.0 × 108 pfu/ml) of phage suspension was spotted onto the plate. Drop was allowed to dry and plate was incubated in inverted position, overnight. According to the degrees of clarity, the spots were differentiated into three categories: clear, turbid, and no reaction.
Organic Solvent Sensitivity of Bacteriophage KPO1K2
Equal volume of bacteriophage (108 pfu/ml) was mixed with appropriate organic solvent viz. chloroform, ethanol, and ether and incubated at room temperature with intermittent shaking. After 1 h, mixture was centrifuged (10,000 rpm/10 min) and phage titer in aqueous phase was estimated by soft agar overlay method against K. pneumoniae B5055 as host. The % inactivation was reported in comparison to phosphate buffer saline suspended phage preparation held at 4°C.
pH Sensitivity of Bacteriophage KPO1K2
For evaluating the stability of bacteriophage at different pH values, pH of the nutrient broth was adjusted with either 1 M HCl or 1 M NaOH to obtain a pH range of 2–12. A total of 1 ml of bacteriophage suspension at a titer of 104 pfu/ml was inoculated into 9 ml of pH adjusted medium to obtain a final concentration of 103 pfu/ml. Preparation was then incubated at 37°C for 1 h. Phage suspension held at pH 7 acted as control. After incubation phage titer was estimated by soft agar overlay method against K. pneumoniae B5055.
Temperature and UV Light Sensitivity
Stability of KPO1K2 toward various temperatures (25, 30, 37, 40, 56, and 60°C) was checked by incubating the phage (108 pfu/ml) at respective temperature for 1 h and then enumerating the phage titer at the end of the incubation. However, optimum temperature for activity of KPO1K2 against K. pneumoniae B5055 was evaluated by exposing the bacteria at a MoI of 0.01 at different temperatures viz. 25, 30, 37, and 40°C for 1 h. After incubation, phage sample was treated with chloroform (1:1) and centrifuged (5000g/5 min). Phage titer in the aqueous phase was estimated by soft agar overlay method. Further, stability of KPO1K2 to UV light was evaluated by exposing the phage suspension (103 pfu/ml) to UV bulb (Sankyo Denki, Japan; G3018, UV-C, λ 253.7 nm).
Stability of the Bacteriophage in Mice
For assessing the in vivo stability of bacteriophage a group of 12 mice (Balb/c, 20–25 g) was injected intraperitoneally (i.p.) with 1 ml of phage preparation (108 pfu/ml). Another group of six mice was injected with heat inactivated phage suspension that acted as −ve control. After appropriate time intervals three mice each (2 test + 1 control), at one time point, were euthanized by cervical dislocation and their organs were removed aseptically into sterile tubes. Phage titers were determined in the supernatant of the homogenized organs. Two mice were injected with sterile normal saline and observed till the duration of experiment. All animal experiments were performed according to the guidelines of the Animal Ethical Committee, Panjab University, Chandigarh, India. At each time point before euthanizing, mice were examined for any signs of illness, lethargy, and their rectal temperature was noted.
Statistical Analysis
Statistical analysis of the data was done by one-way ANOVA using Microsoft Excel Program and P < 0.05 was considered significant.
Results
Ten bacteriophage samples were isolated from effluent water. All were found to produce clear plaques indicating the lytic nature of the bacteriophages. Plaque proper was surrounded by a large halo indicating the production of very high amounts of depolymerase enzyme [13]. From the isolated phages, one with largest halo around the plaque proper was selected for further studies (Fig. 1).
On the basis of spot test to check the host range, phage was found to infect seven out of 25 Klebsiella pneumoniae clinical isolates. Escherichia coli ATCC 25922 was partially sensitive to this bacteriophage giving turbid plaques after overnight incubation while one of the clinical isolates of E. coli was found be sensitive to the partially purified depolymerase enzyme isolated from the same bacteriophage (data not shown). None of the Salmonella or Pseudomonas species showed susceptibility to this phage.
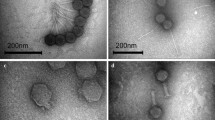
TEM showed that KPO1K2 belonged to Podoviridae family having a short non-contractile tail that is about 10 × 6 nm in its dimensions (Fig. 2). Head of the bacteriophage was found to be icosahedral with pentagonal nature with apex to apex diameter of about 39 nm. Restriction enzyme, EcoRI did not cut the phage DNA while other three restriction enzymes resulted into production of DNA fragments of varying sizes (Fig. 3). On the basis of digestion pattern produced after treatment of DNA with Sau3AI, genome size of the phage was estimated to be approximately 42 kbp which is close to the genome size of T7 phages that varies between 38 kbp and 45 kbp [27].
Agarose gel electrophoresis of bacteriophage DNA digested with restriction enzymes. Label on top of gel shows the respective restriction enzyme used for DNA digestion. M1 and M2 are the λ hind III marker DNA and 1 kb DNA ladder used as reference DNA. Control is the undigested phage DNA. On right side are the markers with their respective band size in kbp
No effect on phage activity was seen after 1 h of incubation with chloroform and diethyl ether. However, after ethanol treatment complete loss of phage activity was seen (Fig. 4b). Similarly phage lost its infectivity completely at pH 3 or below whereas within a pH range of 4–11 no difference in the phage titers with respect to control was observed. At a pH of 12, within 1 h, phage lost approximately 71% of its infectivity and a titer of 1.36 ± 0.10 pfu/ml was observed at the end of incubation. Maximum stability of bacteriophage was found at pH 7.0 (Fig. 4a).
KPO1K2 sensitivity to various pH (a) organic solvents (b) and temperatures (c). For sensitivity to various pH phage preparation held at pH 7.0 acted as control while control for sensitivity to organic solvents and various temperatures was phage preparation held at 4°C in sterile PBS. All values represent the mean of two experiments done in duplicate on different occasions with error bars representing the standard deviation (SD; n = 3)
KPO1K2 was found to be relatively heat stable as over a period of 1 h, between a temperature range of 25–40°C, not a significant loss (P > 0.154) in phage activity was observed. However, at 56°C after 1 h, a 27% reduction in phage activity was observed that showed relative heat stability of bacteriophage (Fig. 4c). While when exposed to 60°C, within first 15 min of incubation >80% phage activity was lost and after 1 h a complete phage inactivation was observed. Moreover, 37°C was found to be the optimum temperature for phage activity even though slight changes in the temperature did not alter the activity of KPO1K2 drastically (data not shown). However, upon exposure to UV light, a complete inactivation of bacteriophage within 80–85 min was observed (data not shown).
Phage growth cycle parameters viz. latent period, eclipse period, rise period, and burst size, were determined from the dynamic change in the number of total and free phages during one replicative cycle. It was found that phage had short latent period of about 15 min with a burst size of ~130–140 pfu/infected cell (Fig. 5). This is a burst size characteristic of lytic phages of family Podoviridae [21]. To further characterize KPO1K2, its structural protein composition was analyzed by SDS-PAGE. Twelve bands could be seen ranging in size from ~120 kDa to 29 kD, with two major proteins being 75 kDa and 43 kDa (Fig. 6).
Within first hour of i.p. inoculation of phage into mice a titer of 4.93 ± 0.32 pfu/ml in blood that increased to a titer of 5.97 ± 0.07 pfu/ml within next 6 h, was observed. However, by 12th hour of inoculation, titer had fallen to 3.51 ± 0.53 pfu/ml. Likewise phage titer in kidney and urinary bladder was also estimated. Titers in kidneys at 1, 6, and 12 h after phage inoculation were 6.9 ± 0.09, 7.84 ± 0.49, and 5.56 ± 0.49 pfu/ml, respectively. However, phage titers in urinary bladder at respective time periods were 6.23 ± 0.64, 6.52 ± 0.12, and 4.27 ± 0.24 pfu/ml (Fig. 7). There was a gradual fall in titer thereafter and ultimately, after 48 h of inoculation, phage became undetectable. Even though there was a difference in the phage titers of blood, kidneys, and urinary bladder but the difference was not significant (P > 0.05). All mice, during the experiment were healthy and active with normal body temperature.
Discussion
The main aim of our study was the isolation and characterization of a lytic bacteriophage with a potential for prophylactic/therapeutic use. Phage with clear plaque and maximum halo size around it was selected for further characterization. Presence of clear plaque is indicative of lytic phage while halo around plaque proper is suggestive of depolymerase activity associated with the bacteriophage. Depolymerase enzyme helps the bacteriophage in establishing the infection in the host, even if latter is producing CPS in abundance that otherwise tends to be a barrier for antimicrobials. A large burst size with short latent period is further suggestive of lytic nature of phage KPO1K2. Finding bacteriophages with broad host range is not uncommon [15]. Scholl et al. [26] isolated lytic bacteriophage with tail fibers that helped the bacteriophage in infecting multiple capsular types. This expression of dual characters helps the phage in extending its host range. In the present study, KPO1K2 not only infected Klebsiella pneumoniae B5055 and some of its clinical isolates but also Escherichia coli ATCC 25922 and one of its five clinical isolates. Since both Klebsiella species and E. coli are known to cause hospital acquired catheter associated UTI, sensitivity of both these bacteria toward a single phage is of added interest. The use of such phages in situations where multiple drug resistant bacteria are encountered can be of help for clinicians.
However, before applying a phage for any therapeutic purpose, determination of its characters is essential. One of the criteria for classification of bacteriophages to a particular group is their morphological and genetic characteristics. Under TEM KPO1K2 was seen to have an icosahedral symmetry with pentagonal outline. This phage belonged to family Podoviridae as it had a small non-contractile tail which is a characteristic of this family. As has been reported earlier head and tail size of KPO1K2 matched with the size range of the T7-like phages [1] suggesting a close resemblance of the isolated phage with T7 phages.
Further evidence to show its closeness to T7 phages is based on its genome size. After digestion with Sau3AI genome size of KPO1K2 was found to be approximately 42 kbps which is close to the genome size of 42,519 bp of T7 phage ΦKMV of Pseudomonas aeruginosa [22] and other T7-like phages [1]. Similarly, two prominent protein bands of 75 and 43 kDa on SDS gel are suggestive of major tail and head proteins, respectively, as has been shown earlier for a T7-like lytic bacteriophage [27]. Bacteriophage KPO1K2 when exposed to various organic solvents was found to be stable in presence of chloroform and ether indicating that this phage could be stored over these agents for prolonged periods without the risk of bacterial contamination. Phage, KPO1K2 was also stable over a wide range of pH (4–11) as no loss in its activity was observed after 1 h of exposure. All these stability studies have implications in the use of this bacteriophage as therapeutic agent for treatment of Klebsiella pneumoniae associated UTI, the purpose for which this phage was isolated. Since this phage did not lose its activity even at extremes of pH hence it can be used for impregnation of urinary catheters for prevention of bacterial biofilms as suggested elsewhere [12, 19]. As at 56°C a 27% reduction in phage activity was observed hence slight exposure of phage impregnated catheters to elevated temperatures would not adversely affect the activity and quality of the catheter. Moreover, since 37°C is the optimum temperature for phage activity, this phage impregnated catheter would be highly effective when placed inside the body.
Phage kinetics for its in vivo stability was performed in mice. When mice were inoculated i.p. with 108 pfu/ml of bacteriophage suspension blood titers of phage reached 4.93 ± 0.32 pfu/ml within 1st hour of injection that increased to 5.97 ± 0.07 pfu/ml at 6th hour of inoculation. However, in an earlier study conducted by Cereveny et al. [9], raised blood titer of bacteriophage was obtained within 1st hour of inoculation and the difference between phage titers at two time points (1st and 6th hour) was not significant. The basic difference in two studies is the route of phage inoculation. It was observed that when phage was given intravenously it accumulated to higher titers in blood in shorter duration than when given intraperitoneally. Since route of phage inoculation is important, hence route of its administration should be chosen with utmost care for the therapy to be effective.
Phage titers in kidney as well as urinary bladder clearly indicated that KPO1K2 accumulated in higher concentrations in these two organs. After that till 12th hour of inoculation, concentration of bacteriophages fell gradually to 3.51 ± 0.53, 5.56 ± 0.49, and 4.27 ± 0.24 pfu/ml in blood, kidney, and urinary bladder, respectively. In blood phage became undetectable by 36th hour whereas in kidney and urinary bladder phage survived and became undetectable only after 48th hour of inoculation. Since, without its host, phage survived for an appreciable duration in, in vivo conditions hence, we speculate that it will act as an excellent antibacterial agent especially in the light of observation that phages are self replicating and will be available in higher concentrations whenever its host is available.
The data obtained in the present study clearly indicates that the isolated phage is a T7-like lytic bacteriophage with a small latent period and large burst size [27] and its relatively longer stability in kidneys as well as urinary bladder makes it a suitable candidate to be tried in Klebsiella pneumoniae associated UTI. Hence focus of our future research would be the application of this characterized bacteriophage in eradicating the catheter associated UTIs caused by Klebsiella pneumoniae both in vivo and in vitro.
References
Ackermann H-W (2005) Bacteriophage classification. In: Kutter E, Sulakvelidze A (eds) Bacteriophages biology, applications. CRC Press, Boca Raton
Ackermann H-W, Dubow MS, Jarvis AW et al (1992) The species concept and its application to tailed phages. Arch Viol 124:69–82
Adams MH (1959) Bacteriophages. Interscience, New York
Ashelford KE, Norris SJ, Fry JC et al (2000) Seasonal population dynamics and interactions of competing bacteriophages and their host in the rhizosphere. Appl Environ Microbiol 66:4193–4199
Ausubel F, Brent R, Kingston RE et al (2001) Current protocols in molecular biology. Wiley and Sons, NY, New York
Bedi MS, Verma V, Chhibber S (2009) Amoxicillin and specific bacteriophage can be used together for the eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Biotechnol (Accepted for publication). doi: 10.1007/s11274-009-9991-8
Bruttin A, Brussow H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 49:2874–2878
Casjens SR (2008) Diversity among the tailed-bacteriophages that infect the Enterobacteriaceae. Res Microbiol 159(2008):340–348
Cerveny KE, DePaola A, Duckworth DH, Gulig PA (2002) Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect Immun 70(11):6251–6262
Chhibber S, Kaur S, Kumari S (2008) Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 57:1508–1513
Comeau AM, Tétart F, Trojet SN, Pre`re M-F, Krisch HM (2007) Phage-Antibiotic Synergy (PAS): b-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2(8):e799. doi:10.1371/journal.pone.0000799
Danese PN (2002) Antibiofilm approaches: review prevention of catheter colonization. Chem Biol 9:873–880
Geyer H, Himmelspach K, Kwiatkowski B et al (1983) Degradation of bacterial surface carbohydrates by virus-associated enzymes. Pure Appl Chem 55:637–653
Gilbert P, Das J, Foley I (1997) Biofilm susceptibility to antimicrobials. Adv Dent Res 11(1):160–167
Goodridge L, Gallaccio A, Griffiths MW (2003) Morphological, host range, and genetic characterization of two coliphages. Appl Environ Microbiol 69(9):5364–5371
Gorski A, Weber-Dąbrowska B (2005) The potential role of endogenous bacteriophages in controlling invading pathogens. Cell Mol Life Sci 62:511–519
Hendrix RW (2002) Bacteriophages: evolution of the majority. Theor Popul Biol 61:471–480
Holloway BW, Krishnapillai V, Morgan AF (1970) Chromosomal genetics of Pseudomonas. Microbiol Rev 43(1):73–102
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144(2):117–126
Kumari S, Harjai K, Chhibber S (2008) Efficacy of bacteriophage treatment in murine burn wound infection induced by Klebsiella pneumoniae. J Microbiol Biotechnol (published online December). doi:10.4014/jmb.0808.493
Kutter E, Sulakvelidze A (2005) Bacteriophages biology and applications. CRC Press, Boca Raton
Lavigne R, Burkal’tseva MV, Robben J et al (2003) The genome of bacteriophage phi KMV, a T7-like virus infecting Pseudomonas aeruginosa. Virology 312:49–59
Maniatis T, Sambrook J, Fritsch EF (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Miedzybrodzki R, Switala-Jelen K, Fortuna W et al (2008) Bacteriophage inhibition of reactive oxygen species generation by endotoxin-stimulated polymorphonuclear leukocytes. Virus Res 131:233–242
Pajunen M, Kiljunen S, Skurnik M et al (2000) Bacteriophage phi YeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphages T3 and T7. J Bacteriol 182:5114–5120
Scholl D, Rogers S, Adhya S, Merril CR (2001) Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J Virol 75(6):2509–2515
Sillankorva S, Neubauer P, Azeredo J (2008) Isolation and characterization of a T7-like lytic phage for Pseudomonas fluorescens. BMC Biotechnol 8:59–70
Sutherland IW (2001) Biofilm polysaccharide: a strong and sticky framework. Microbiology 147:3–9
Suttle CA (2007) Marine viruses—major players in the global ecosystem. Nat Rev Microbiol 5:801–812
Vinodkumar CS, Neelagund YF, Kalsurmath S (2005) Bacteriophage in the treatment of experimental septicaemia mice from a clinical isolate of multidrug resistant Klebsiella pneumoniae. J Commun Dis 37:18–29
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182(10):2675–2679
Weld R (2000) Bacteriophage on the menu. Pharm Sci Technol Today 3(12):404–405
Yamamoto KR, Alberts BM, Benzinger R et al (1970) Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40:734–744
Acknowledgment
This study was supported by the Indian Council of Medical Research (ICMR). We also thank Dr. Rupinder Tiwari for the facilities used in his laboratory pertaining to restriction digestion experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, V., Harjai, K. & Chhibber, S. Characterization of a T7-Like Lytic Bacteriophage of Klebsiella pneumoniae B5055: A Potential Therapeutic Agent. Curr Microbiol 59, 274–281 (2009). https://doi.org/10.1007/s00284-009-9430-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9430-y