Abstract
Arginine deiminase (ADI), an arginine-degrading enzyme, has been studied as a potential anti-cancer agent in clinical trials for the treatment of arginine-auxotrophic tumors, such as hepatocellular carcinomas (HCCs) and melanomas. The arcA gene encoding ADI was cloned from a recently isolated strain Pseudomonas plecoglossicida CGMCC2039. The nucleotide sequence of ADI comprises an ORF of 1,254 bp encoding 417 amino acids. The deduced ADI protein sequence has a calculated molecular weight of 46.5 kDa and shows 97% and 85% identity to ADIs from P. putida and P. aeruginosa, respectively. The arcA from P. plecoglossicida CGMCC2039 was expressed in Escherichia coli BL21 with a N-terminal His6-tag, and purified to homogeneity. A molecular mass of approximate 49 kDa was confirmed by SDS-PAGE analysis and specific activity was determined to be 4.76 U/mg (pH 6.0 and 37°C). In vivo activity study showed that the rADI could effectively inhibit H22 tumor growth at a total dose of 5 U/mouse over a 2-week dosing period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
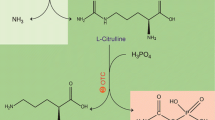
Arginine deiminase (ADI; EC 3.5.3.6) belongs to a guanidino group-modifying enzyme superfamily and catalyzes the irreversible hydrolysis of arginine to citrulline and ammonia [16]. ADI genes from different microorganisms, including Streptococcus sanguis [2], Mycoplasma arginini [10], Pseudomonas aeruginosa [12], and Lactococcus lactis ssp. Lactis [8], have been cloned and expressed in Escherichia coli strains to investigate the potential pharmaceutical activity of ADI and its roles in the complex arginine metabolism pathways [11].
Previous studies suggested that ADI could be a potential therapeutic agent against arginine-auxotrophic tumors, especially hepatocellular carcinomas (HCCs) and melanomas, due to its anti-proliferative activity [4, 5, 11, 15]. It was also demonstrated that ADI could induce cell cycle arrest and apoptosis of human leukemia cells [6] and have anti-angiogenic effect on endothelial cells [13]. Currently, clinical trials of ADI-PEG-20 for the treatment of HCCs (Phase III) and melanomas (Phase I/II) are being investigated [7, 15].
In our preceding study, a Pseudomonas plecoglossicida GMCC2039 strain which exhibited remarkable ADI activity and in vitro anti-tumor activity was isolated and characterized [9, 17]. In this study, the arcA gene from P. plecoglossicida CGMCC2039 was cloned, sequenced, and expressed in functional form in E. coli BL21 (DE3). The His-tagged ADI was purified to homogeneity and characterized in respect to temperature optimum, pH activity, and specific activity for arginine conversion. The effectiveness of rADI was further evaluated in mice with implanted H22 tumor, in which an inhibition rate of 82.3% was observed when a total dose of 5 U/mouse was administrated over a 2-week time course.
Materials and Methods
Strain and Culture Conditions
P. plecoglossicida CGMCC2039 was isolated from Wuxi canal (Jiangsu, China) as the source for arcA isolation, and was cultured in fermentation medium [0.3% (w/v) yeast extract, 0.3% (w/v) peptone, 1.2% (w/v) Na2HPO4 · 12H2O, 0.3% (w/v) NaH2PO4 · 2H2O, 0.5% (w/v) l-arginine, pH 7.0] containing 1.5% (w/v) glucose for 24 h at 30°C [9].
Cloning and Sequence Analysis of arcA
Genomic DNA of P. plecoglossicida CGMCC2039 was prepared using phenol/chloroform extraction. For cloning arcA from P. plecoglossicida CGMCC2039, two primers (forward, 5′-ATGTCCGCTGAAAAACAGAAGTACG-3′; reverse 5′-TTAGTAGTTGATCGGGTCGCGCACG-3′) were derived from the 5′ and 3′ sequences of arcA in P. putida KT2440 (NCBI Gene ID: 1044932). PCR amplification was performed using Ex-Taq DNA polymerase (TaKaRa, Japan) and a PTC-200 thermocycler (Bio-Rad, USA). The amplified fragment was inserted into pMD18-T Vector (TaKaRa, Japan), and the nucleotide sequence of ADI was then determined by TaKaRa Biotechnology (Dalian) Co., Ltd.
Expression and Purification of Recombinant ADI
To facilitate the purification procedure, pET28a was selected as the expression vector, in order to incorporate a N-terminal His-Tag in the fusion protein. E. coli BL21(DE3) was used as the expression host. The arcA gene was amplified from the genomic DNA from P. plecoglossicida CGMCC2039 by PCR using primers (forward, 5′-GAAGTCCATATGTCCGCTGAAAAACAGAAG-3′; reverse 5′-AGTGGTCTCGAGTTAGTAGTTGATCGGGTC-3′) in which restriction sites Nde I and Xho I (underlined) were included. The amplified fragment was subcloned into pMD18-T vector, before cloning into the pET28a vector using Nde I and Xho I as restriction sites. The recombinant plasmid was designated as pET28a-arcA and was then transformed into E. coli BL21(DE3) using CaCl2 method [14].
E. coli BL21(DE3) cells harboring pET28a-arcA were incubated in a 250-ml shake flask containing 50 ml LB medium supplemented with 30 μg/ml Kanamycin at 37°C and 180 rpm overnight. One milliliter of the overnight culture was inoculated into a 500-ml shake flask with 100 ml of fresh LB medium and was incubated under the same conditions as above. At 0.6 OD600, IPTG was added to a final concentration of 0.4 mM to induce the expression of arginine deiminase. After 4 h of induction, the cell pellets were collected (6,000g, 10 min at 4°C), washed twice with saline water, and resuspended in 20 ml sodium phosphate buffer (50 mM, pH 7.4).
The N-terminal His-Tag of the recombinant ADI (rADI) was used for purification by nickel-chelated affinity column chromatography. The above mentioned cell suspension in phosphate buffer was sonicated on ice (400 w, 200 × 5 s pulse with 5 s rest in between), and the clear supernatant was collected by centrifugation (8,000g, 10 min at 4°C). The supernatant was filtered (cellulose membrane, 0.45 μm; Xingya purification material Co., Shanghai, China), and loaded onto a 1 ml pre-packed nickel-chelated agarose gel column (Weishi-Bohui Chromtotech Co., Beijing, China). The His-tagged rADI was eluted by elusion with imidazole (200 mM) and stored in −20°C freezer for future use after dialysis against sodium phosphate buffer (50 mM, pH 7.4, V enzyme:V buffer = 1:100, exchange buffer once a half-an-hour six times). The expression and purification of rADI were analyzed by 12% SDS-PAGE. The protein bands were visualized by staining with Coomassie brilliant blue. One-StepTM His-Tag Western Kit (Genscript Co., USA) was used for western blot analysis.
rADI Activity Assay
The rADI activity was determined by the amount of l-citrulline produced from l-arginine [1]. In brief, cell pellets from 1 ml of culture were collected and washed with saline water. The washed cells were treated with 1 ml of 1 mg/ml cetyltrimethyl ammonium bromide (CTAB) for 10 min, and collected by centrifugation. Then the cells were resuspended in a reaction mixture, containing 0.2 M l-arginine hydrochloride and 0.2 M sodium phosphate buffer (pH 6.0), in a final volume of 1 ml. As for purified rADI, 100 μl rADI solution was added into the same reaction mixture in a final volume of 1 ml. The reaction mixture was incubated at 37°C for 30 min. The ADI activity was determined by measuring the formation of l-citrulline from l-arginine as described in our previous study [9]. One unit (U) of ADI activity is defined as the amount of enzyme that is required for converting 1 μmol of l-arginine into 1 μmol of l-citrulline per min under the assay conditions. The amount of protein was determined by the Bradford method using bovine serum albumin as standard.
In Vivo Inhibition of Tumor Growth by rADI
Female Qunming mice (4–6 weeks old, 18–22 g weight) were obtained from Shanghai Silaike Experimental Animal Center. Approximately, 2×106 H22 hepatoma cells were injected into the hind limb of the mice. After 24 h of implantation, the tumor-implanted mice were randomly divided into three drug (rADI) groups and two control (drug and blank) groups with 10 mice each, which were treated once every 3 days with total doses of 2.5 U/mouse, 5 U/mouse, 10 U/mouse, 1.0 mg fluorouracil/mouse (as drug control), and saline (as blank control) in 2 weeks, respectively. Five mice without tumor implant were used as normal control. After 2 weeks, the tumor was harvested and weighed. The weights of liver and spleen were also measured. The significant difference was evaluated by independent t-test analysis.
Results
Sequence Determination and Homology Analysis
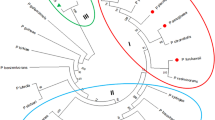
A 1,254-bp fragment encoding the arcA gene from P. plecoglossicida CGMCC2039 was deposited in the GenBank database under Accession No. EU030267. The derived ADI amino acid sequence resulted in a protein with calculated molecular mass of 46.5 kDa, exhibiting the highest identity of 97% with ADI from Pseudomonas putida (EAX15803; see BLAST analysis in Fig. 1). Alignment with ADIs from other microorganisms, including Pseudomonas aeruginosa (X14694, 85%), Lactococcus lactis ssp. Lactis (DQ364637, 30%), and Mycoplasma arginini (X54141, 29%) (Fig. 1), indicated they all shared the same conserved motif and residues [3, 8], in spite of the low homology demonstrated by Lactococcus lactis ssp. Lactis and Mycoplasma arginini. Specifically, a catalytic triad consisting of the same residues (Cys-His-Glu) and characteristic substrate-binding sites are conserved (Fig. 1).
Alignment of ADI protein sequences from P. plecoglossicida CGMCC2039 (EU030267), P. putida (EAX15803), P. aeruginosa (X14694), L. lactis ssp. lactis (DQ364637), and M. arginini (X54141). Key amino acids denoted with asterisks are involved in binding of the substrate and amino acids marked with dots indicated the catalytic triad (Cys-His-Glu)
Expression and Purification of rADI
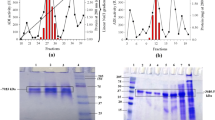
Under the control of the strong T7 promoter, the arcA gene was over-expressed in E. coli BL21(DE3) as indicated by SDS-PAGE. Under IPTG induction, a strong band was detected at approximately 49 kDa (Fig. 2a, Lane 2), whereas no noticeable band was observed without IPTG induction (Fig. 2a, Lane 3). No expression was obtained, as expected, in an E. coli host carrying only the empty vector (negative control; Fig. 2a, Lane 4). The SDS-PAGE results were consistent with the activity assays. A basal expression of 0.032 U/mg DCW (dry cell weight) was detected without IPTG induction, comparing with 0.561 DCW U/mg with IPTG induction. No detectable activity was observed for the negative control. The supernatant and sedimentary fractions after sonication were also analyzed by SDS-PAGE (Fig. 2a, Lane 5, 6), and a dense band around 49 kDa was observed in the sedimentary sample. The result suggested that only a small portion of rADI is water soluble, and most of the expression product was formed as inclusion body likely due to the strong T7 promoter system. In fact, majority of the protein remained as inclusion bodies after our attempts to optimize the expression conditions such as induction temperature and IPTG concentration. Western blotting analysis for detecting His-tagged recombinant protein was performed, and single band was visualized at the right position for both IPTG induced culture and purified rADI (Fig. 2b). His-tagged rADI was one-step purified to homogeneity by nickel-chelated affinity column chromatography with a specific activity of 4.76 U/mg and yield of 72.48%. The purity of the rADI sample was confirmed by SDS-PAGE analysis as indicated by a single band at molecular mass of 49 kDa (Fig. 2a, Lane 1).
SDS-PAGE and Western Blotting analysis of purified rADI and its expression. Panel (a), M: molecular mass standards; 1: purified rADI; 2–3: E. coli BL21 harboring pET28a-arcA plasmid induced by IPTG (2) and without IPTG (3); 4: E. coli BL21 harboring pET28a plasmid as negative control; 5–6: supernatant and sedimentary fractions after sonication. Panel (b), lanes 1 and 2 are the same as lanes 1 and 2 in panel (a), directed specifically against the 6× histidine tag on the recombinant protein using One-StepTM His-Tag Western Kit (Genscript Co., USA). Around 10–20 μg of protein sample was loaded in each lane
Enzymatic Properties of rADI
The effects of pH and temperature on rADI from P. plecoglossicida CGMCC2039 are shown in Fig. 3. The rADI exhibited higher activity under the acidic condition with optimal pH of 6.0 and temperature of 37°C (Fig. 3a, b). Only 30% and 10% of the activity were retained at pH 7.0 and at temperatures higher than 55°C, respectively (Fig. 3a, b). The thermostability study indicated that rADI was sensitive to high temperatures. Specifically, the activity of rADI decreased to undetectable level after being incubated at 50°C for 40 min (Fig. 3c).
Enzymatic properties of purified rADI. The results are mean values from triplet experiments with bars indicating ±SD. a pH dependency of rADI. The activities are shown as percentages relative to that at 37°C. Optimum pH of rADI was determined using the buffer solutions of 100 mM citric acid buffer (pH 4.0–5.0), 200 mM sodium phosphate buffer (pH 5.5–8.0) at 37°C. b Temperature dependency of rADI activity. The activities are shown as percentages relative to that measured at pH 6.0. Optimum temperature of the purified rADI was measured from 4 to 60°C in 200 mM sodium phosphate buffer (pH 6.0). c Thermostability of rADI. For the thermostability determination, the rADI was incubated at 30°C (square), 37°C (circle), and 50°C (triangle) in 200 mM sodium phosphate buffer (pH 6.0)
Inhibition of Tumor Growth by rADI In Vivo
The in vivo anti-tumor activity of rADI was evaluated in murine models bearing hepatoma H22 cell line. The implanted mice were treated with rADI every three days in total doses of 2.5, 5, and 10 U/mouse (designated as rADI-2.5, rADI-5, rADI-10, respectively) in 2 weeks. As shown in Table 1, the total dose of 5 U/mouse decreased tumor mass significantly. Tumor growth inhibition rate of 82.3% was observed, which is close to the fluorouracil-treated (drug control) group (93.0%). It was noticed that low dose (2.5 U/mouse) had no appreciable anti-tumor activity against H22 since the tumor weight was essentially the same as the saline-treated group. High dose of rADI (10 U/mouse) exhibited moderate inhibitory effect on tumor growth. The results indicate that, instead of a simple accumulative effect, the relationship between the dose of rADI and its in vivo anti-tumor activity could be much complicated. In addition, liver and spleen indexes ((liver/spleen weight/body weight) × 100%) are often used to evaluate the effects of therapeutic agents on the immunoreactions of internal organs. Among three doses, the results demonstrated that the liver and spleen indexes of rADI-5 group were the lowest and were also closer to the drug control group than the other two, while the indexes of rADI-10 were the highest (data not shown). Our study suggests the effective doses of rADI might be closely related to the immunoreactions in vivo, which await further studies.
Discussion
In this study, the arcA gene that encodes arginine deiminase was cloned from a newly isolated strain P. plecoglossicida CGMCC2039 and expressed in E. coli BL21(DE3). The alignment of deduced amino acid sequence of arcA with those from other microorganisms showed they shared the same catalytic triad (Cys-His-Glu) and substrate-binding residues (Fig. 1). However, their specific activities differ substantially, varying from 0.115 to 140.3 U/mg [2, 8, 10, 12], which could be associated with different origins of the ADI genes, as well as their various subunit structures [11].
The rADI seemed sensitive to both high temperature and physiological or alkali pH conditions, reserving less than half activity at 50°C and pH 7.0. An acidic optimal pH of 6.0 was observed, which is the same as ADIs from P. putida and M. hominis. Actually, acidic to neutral pH optima ranging from 5.6 to 7.6 have been reported for ADIs from different origins [11]. Optimum temperature for rADI was determined to be 37°C, different from those reported from many other microorganisms (E. gracilis, 30°C; H. salinarium, 40°C; P. putida, M. arginini, 50°C; L. lactis ssp. lactis 60°C) [8]. And the rADI was inactivated rapidly at high temperature; over 90% of the activity was lost at 55°C or higher.
To test the effectiveness of rADI in inhibiting tumor growth in vivo, hepatoma H22 implanted mice model were used. At a total injection of 5 U/mouse in 2 weeks, the inhibition rate reached 82.3%, which was the highest among the three doses. Lower dose of rADI (2.5 U/mouse) gave only 2.8% inhibition rate, which might largely be due to its poor stability under physiological temperature (Fig. 3c). The inhibition rate at a higher dose of 10 U/mouse (22.3%) was much lower than that of 5 U/mouse (82.3%), indicating a complex correlation between the dose of rADI and its anti-tumor efficacy. In addition, immunoreactions were evident when higher liver and spleen indexes were observed for rADI-10 than that of the lower dose group rADI-5. Our preliminary studies therefore confirmed that, besides its arginine-deprivation activity, high level of native rADI could trigger immunoreactions of internal organs (such as liver and spleen) and may compromise its anti-tumor effect, which is consistent with the marked immune response observed with non-PEGylated ADI in the preclinical models [7].
In current clinic studies, ADI from M. hominus has been exclusively used as an anti-tumor drug for HCCs and melanomas in its PEGylated form, reportedly due to its most optimal combination of physiological pH optimum and highest affinity for arginine among ADIs purified from different microbes [4, 5, 7]. Our ADI from P. plecoglossicida undoubtedly represents a novel source of a promising cancer therapy agent for drug development. Furthermore, the availability of this recombinant protein will facilitate the pursuit of the anti-tumor mechanism of this enzyme to combat cancers. In future studies, we aim to improve the fraction of functional ADI expression using strategies such as investigation of alternative expression systems capable of secreting proteins, development of an efficient protocol for renaturation of ADI inclusion bodies. Our further studies would also focus on boosting rADI’s anti-cancer potential by protein engineering of ADI for improved activity under neutral pH, and its chemical modifications (such as PEG formulation) and human serum albumin (HSA) fusion for prolonged serum half-life.
References
Boyde TRC, Rahmatullah M (1980) Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem 107:424–431
Burne RA, Parsons DT, Marquis RE (1989) Cloning and expression in Escherichia coli of the genes of the arginine deiminase system of Streptococcus sanguis NCTC 10904. Infect Immun 57:3540–3548
Das K, Butler GH, Kwiatkowski V et al (2004) Crystal structures of arginine deiminase with covalent reaction intermediates; implications for catalytic mechanism. Structure 12:657–667
Ensor CM, Holtsberg FW, Bomalaski JS et al (2002) Pegylated arginine deiminase (ADI-SS PEG20, 000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 62:5443–5450
Feun L, Savaraj N (2006) Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs 15:815–822
Gong H, Zolzer F, von Recklinghausen G et al (2000) Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia 14:826–829
Izzo F, Marra P, Beneduce G et al (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from Phase I/II studies. J Clin Oncol 22:1815–1822
Kim JE, Jeong DW, Lee HJ (2007) Expression, purification, characterization of arginine deiminase from Lactococcus lactis ssp. lactis ATCC 7962 in Escherichia coli BL21. Protein Expr Purif 53:9–15
Liu YM, Sun ZH, Ni Y et al (2008) Isolation and identification of an arginine deiminase producing strain Pseudomonas plecoglossicida CGMCC2039. World J Microb Biot 24:2213–2219
Misawa S, Aoshima M, Takaku H et al (1994) High-level expression of Mycoplasma arginine deiminase in Escherichia coli and its efficient renaturation as an anti-tumor enzyme. J Biotechnol 36:145–155
Ni Y, Schwaneberg U, Sun ZH (2008) Arginine deiminase, a potential anti-tumor drug. Cancer Lett 261:1–11
Oudjama Y, Tricot C, Stalon V et al (2002) Overexpression, purification, crystallization and preliminary X-ray crystallographic analysis of Pseudomonas aeruginosa l-arginine deiminase. Acta Crystallogr D Biol Crystallogr 58:2150–2152
Park IS, Kang SW, Shin YJ et al (2003) Arginine deiminase: a potential inhibitor of angiogenesis and tumor growth. Br J Cancer 89:907–914
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shen LJ, Shen WC (2006) Drug evaluation: ADI-PEG-20—a PEGylated arginine deiminase for arginine-auxotrophic cancers. Curr Opin Mol Ther 8:240–248
Shirai H, Blundell TL, Mizuguchi K (2001) A novel superfamily of enzymes that catalyze the modification of guanidino groups. Trends Biochem Sci 26:465–468
Sun ZH, Zheng P, Ni Y et al (2007) An arginine deiminase producing strain and its application. Chinese patent (Application No. 200710107822.X)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, Y., Li, Z., Sun, Z. et al. Expression of Arginine Deiminase from Pseudomonas plecoglossicida CGMCC2039 in Escherichia coli and Its Anti-Tumor Activity. Curr Microbiol 58, 593–598 (2009). https://doi.org/10.1007/s00284-009-9376-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9376-0