Abstract
Findings show 21 fungal isolates belonging to eight genera recovered from Egyptian soils that have the potential to attack l-methionine under submerged conditions. Aspergillus flavipes had the most methioninolytic activity, giving the highest yield of l-methioninase (10.78 U/mg protein), rate of methionine uptake (93.0%), and growth rate (5.0 g/l), followed by Scopulariopsis brevicaulis and A. carneus. The maximum l-methioninase productivity (11.60 U/mg protein) by A. flavipes was observed using l-methionine (0.8%) as an enzyme-inductive agent and glucose (1%) as a co-dissimilated carbon source. A significant reduction in l-methioninase biosynthesis by A. flavipes was detected using carbon-free medium, suggesting the lack of ability to use l-methionine as a carbon and nitrogen source. Potassium dihydrogen phosphate (0.25%), the best source of phosphorus, favors enzyme biosynthesis and enhances the level of methionine uptake by A. flavipes. The maximum l-methioninase productivity (12.58 U/mg protein) and substrate uptake (95.6%) were measured at an initial pH of 7.0.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-methioninase (l-methionine-α-deamino-γ-mercaptomethane-lyase, E.C 4.4.1.11) is a pyridoxal-l-phosphate-dependent enzyme that catalyze the conversion of l-methionine to methanethiol, α-ketobutyrate and ammonia via oxidative deamination and demethiolation processes [40] as follows:

Much attention has been paid to l-methionine-lyase since it was reported extensively as an antitumor agent against numerous malignant cell lines (breast, lung, colon, kidney, and glioblastoma) [9, 17, 25, 45, 46]. The antitumor activity of l-methioninase elaborates from the absence of tumor cells to methionine-synthase [17]. Consequently, these cells are auxotrophic for l-methionine, depending absolutely on the exogenous supply of l-methionine for their survival and proliferation [26], whereas normal cells are relatively resistant to exogenous l-methionine restriction because it contains active methionine synthase [4]. Moreover, l-methioninase has a major role in food industries by imparting a distinctive aroma to many traditional fermented foods including cheeses via degradation of l-methionine that releases volatile sulphur compounds [12].
l-methioninase was recovered from the culture filtrates of some rumen bacteria [30] including Clostridium sp. [51], Escherichia coli [34] and Aeromonas sp. [33]. Furthermore, properties of Pseudomonas putida and P. ovalisl-methioninase were studied extensively [20, 21]. Unfortunately, the therapeutic efficiency of bacterial l-methioninase has rarely occurred without some evidence of toxicity and immunogenic reactions, especially with regard to multiple doses, which may restrict their clinical utility [47]. Various attempts have been made to overcome the high immunogenicity and rapid clearance of l-methioninase from plasma including immobilization with biocompatible polymers such as polyethylene glycol (pegylation) that one of the most applicable techniques for providing l-methioninase with less immunogenic properties [24].
On the other hand, the production of l-methioninase by eukaryotes including fungi is the most promising biotechnological process. This results in therapeutic enzymes with fewer immunogenic and allergic reactions, which may be attributed to the higher specificity to their substrates compared with the substrate analogues, displaying a less troublesome during the course of tumor therapy [18]. It is noteworthy that reports describe l-methioninase in the culture filtrates of a few yeasts including Geotrichum candidum, Debaromyces hansenii and Saccharomyces cerevisiae [7]. As it appeared from the documented literature, a few isolates of filamentous fungi are reported to be methionine decomposers including Shizophyllum commune [11], Microsporum gypseum, Scopulariopsis brevicaulis [44], Aspergillus niger, and Aspergillus sp. RS-1a [40].
Practically no comprehensive publications have documented the potential of filamentous fungi for l-methioninase production and the culture conditions regulating l-methioninase productivity. Therefore, in this context, the methioninolytic potentials of soil fungi were screened. The nutritional culture conditions were optimized to maximize the enzyme yield by the experimental fungal isolate under submerged conditions.
Materials and Methods
Materials
l-methionine, l-glycine, l-asparagine, l-glutamine, l-glutamic acid, trichloroacetic acid, sodium nitroprusside, and Nessler reagent (HgCl2, KI and NaOH) were purchased from Sigma Chemical (St. Louis. MO, USA). Folin reagent was obtained from LOBA Chemie (Mumbai, India). All the other chemicals were of analytical grade.
Isolation and Identification of Methioninolytic Fungi
Using the protocol of Johnson et al. [23], l-methionine-decomposing fungi were isolated from different soils from Sharkia province, Egypt. Methionine-glucose medium [40] contains methionine (5 g/l), glucose (10 g/l), K2HPO4 (1 g/l), KH2PO4 (1 g/l), MgCl2. 6H2O (0.5 g/l), CaCl2. 2H2O (0.1 g/l), FeCl3. 6H2O (0.02 g/l), ZnCl2 (0.02 g/l), and agar-agar (20 g/l), all dissolved in 1 l of distilled water and used as an isolation medium. The pH of the medium was adjusted to 7.0. The fungal plates were incubated at 28 ± 1°C for 10 days, and the developed fungal isolates were purified on the same basal medium. The purified fungal isolates were identified according to the universally accepted keys adopted by Rifai [38], Ellis [15], Raper and Fennell [37], Booth [8], Pitt [36], Domsch et al. [13], Lund [29], Abarca et al. [1], and Samson et al. [42].
The fungal cultures were included in the Mycological Culture Collection, Faculty of Science, Zagazig University, Egypt. These cultures were maintained on potato-dextrose agar slants [5].
Fermentation Media and Culture Conditions
The conidial suspension was prepared by injecting 10 ml of sterilized saline solution (0.85%) into a 7-day-old slant of each fungus [16]. The fungal isolates were screened for their l-methioninase productivities using methionine-glucose liquid medium [40]. This involved dispensing 50 ml of fermentation media in 250-ml Erlenmeyer conical flasks and inoculating the media with a 1-ml spore suspension of each fungal isolate. The submerged cultures were incubated at 28 ± 1°C for 8 days in a shaker incubator (New Brunswich Scientific, Edison, NJ, USA) at 130 rpm.
Optimization of Some Nutritional Parameters for Enzyme Production Using Submerged Fermentation Processes
To maximize the l-methioninase productivity by the selected fungal isolate, various chemical parameters were optimized. Several carbon sources, namely, xylose, glucose, arabinose, mannitol, sorbitol, citric acid, potassium oxalate, and sodium benzoate (1% w/v), sucrose, lactose, and maltose (0.5%w/v), and cellulose and starch (0.25% w/v) were supplemented to the basal medium as cometabolic agents for l-methionine dissimilation. The l-methionine free medium was supplemented with various nitrogen sources, namely, l-asparagine, l-glutamine, l-glutamic acid, arginine, tyrosine, glycine, urea, beef extract, yeast extract, malt extract, ammonium oxalate, ammonium chloride, peptone, sodium nitrate, ammonium sulphate, and ammonium molybdate according to their equivalent molecular weights to evaluate their effect on l-methioninase induction by the fungal isolate. Also, the effect of various phosphorus sources (e.g., K2HPO4, KH2PO4, NaH2PO4, Na2HPO4) with different concentrations on the enzyme biosynthesis was investigated. In addition, the effect of the initial pH value (2–9) of production medium on methionine uptake and the productivity of l-methioninase by the experimental organism was investigated. After the fermentation period, the culture was centrifuged at 5,000 rpm for 10 min at 4°C, and the supernatants were used as a crude enzyme.
Methioninase Assay
Routinely, l-methioninase activity was assayed by direct Nesslerization according to the method of Thompson and Morrison [50] with some modifications. The standard reaction system contains 1 ml of 1% l-methionine in citrate phosphate buffer (pH 7.0), 0.1 ml of pyridoxal phosphate, and 1 ml of crude enzyme. The reaction system was incubated at 30°C for 1 h. The enzymatic activity was stopped by adding 0.5 ml of 1.5 mol/l trichloroacetic acid. The system was centrifuged at 5,000 rpm for 5 min to remove the precipitated protein. The released ammonia was determined using 0.5 ml of Nessler reagent, and the developed colored compound was measured at 480 nm using the Spekol-spectrocolorimeter. Enzyme and substrate blanks were used as controls. One unit of l-methioninase was defined as the amount of enzyme that liberates ammonia at 1 μmol/h under optimal assay conditions. The specific activity of l-methioninase was expressed as the activity of enzyme in terms of units per milligram of protein.
Determination of Extracellular Protein
The protein concentration of the crude enzyme preparation was estimated by Folin reagent according to the protocol of Lowry et al. [28] using bovine serum albumin as the standard. The enzyme concentration was expressed in terms of milligrams per milliliter of crude extract.
Determination of Methionine
The residual methionine of culture filtrate was determined on the basis of the thioether group according to the method of Hess and Sullivan [19] with some modifications. Using this method, 1 ml of the supernatant was mixed with 0.5 ml of 3% glycine, 1 ml of 2% sodium nitroprusside, and 0.5 ml of 1 N NaOH. The mixture was incubated in a water bath at 40°C for 15 min., then chilled in an ice bath for 5 min. Next, 1 ml of a 1:9 (v/v) mixture of HCl:H3PO4 was added with vigorous shaking for 5 min. The developed color was measured spectrophotometrically at 530 nm. The methionine concentration was determined from the standard curve of methionine prepared under the same conditions. The rate of methionine uptake was expressed as the amount of consumed methionine/initial methionine concentration ×100.
Biomass Determination
After the fermentation process, the cultures were centrifuged at 5,000 rpm for 10 min at 4°C followed by filtration through Whatman no. 1 filter paper. The cell pellets were washed with distilled water and dried at 80°C until a constant weight was achieved. The dry biomass was expressed as grams per liter of fermentation medium.
Statistical Analysis
All the bioprocesses were performed in triplicate, and a one-way ANOVA test was used to estimate the data mean and standard deviation [43].
Results and Discussion
Methioninolytic Potentiality of Some Soil Fungi
Using l-methionine-glucose agar medium, 21 fungal isolates were recovered from soil samples and identified using the universally accepted keys (materials and methods). These species belonged to eight fungal genera, namely, Aspergillus, Penicillium, Fusarium, Cladosporium, Scopulariopsis, Humicola, Trichoderma, and Mucor. The genus Aspergillus had the most frequencies, representing nine species belonging to five groups according to Raper and Fennel [37] as follows: A. flavus group (A. flavus, A. oryzae, A. subolivaceous, A. tamarii and A. parasiticus), A. candidus group (A. carneus), A. flavipes group (A. flavipes), A. ochraceous group (A. ochraceous), and A. niger group (A. niger). The genus Penicillium was represented by four species belonging to the section Asymmetrica, according to Pitt [36], one belonging to the subsection Divaricata (P. egyptiacum), and the other three belonging to Volutina (P. digitatum, P. notatum, P. citrinum). The genus Fusarium also was represented by three species of the following three sections: Martiella (F. solani), Elegans (F. oxysporum), and Arachnites (F. nivale), whereas the other five genera were represented by one species each as follows: Cladosporium oxysporum, Scopulariopsis brevicaulis, Humicola fuscoatra, Trichoderma koningii, and Mucor racemosus.
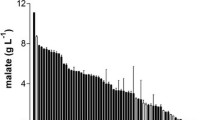
The screening profile (Table 1) shows the variability of l-methioninase production among the different fungal isolates. The maximum l-methioninase productivity (10.78 U/mg protein), the l-methionine uptake (93%), and the biomass (5 g/l) were obtained by Aspergillus flavipes followed by Scopulariopsis brevicaulis, A. carneus, Penicillium notatum, Fusarium solani, F. nivale, and F. oxysporum. The lowest enzyme productivity and uptake of methionine were detected in the culture filtrates of A. parasiticus followed by A. ochraceous, A. tamarii, A. subolivaceous, A. oryzae, and Cladosporium oxysporum. These results clearly elucidate the productivity of l-methioninase by different fungal isolates directly proportional to the rate of l-methionine uptake but may not to the biomass of the microorganism. No published reports have described this screening system for l-methioninase production by fungi. Many microorganisms, including fungi, attack methionine but do not grow on it, perhaps because of their inability to methabolize the deaminated (α-ketomethionine) and demethiolated (α-keto-butyric acid and methanthiol) residues of l-methionine. The inability of filamentous fungi to grow on l-methionine may be partially overcome by the use of a growth-supporting organic compound such as glucose or an equivalent carbohydrate designated as a co-dissimilator [40]. In connection with our results, several fungal species including Aspergillus sp. RS-1a [40], Microsporum gypseum, Scopulariopsis brevicaulis, A. niger [44], and Shizophyllum commune [11] have the ability to hydrolyze l-methionine under co-metabolic conditions.
From this preliminary screening experiment, it appeared that Aspergillus flavipes was the most promising fungal isolate for the production of l-methioninase. Therefore, it was selected for subsequent experimentation to increase its enzyme productivity.
Induction of l-Methioninase by Aspergillus flavipes Using Various Nitrogen Sources
The influence of different nitrogen sources on the induction of l-methioninase by A. flavipes was evaluated (Fig. 1). Among the different nitrogen sources, l-methionine was observed to be the optimum inducer of l-methioninase biosynthesis (10.78 U/mg protein) by A. flavipes, followed by l-glutamine (4.8 U/mg protein). The other tested nitrogenous compounds had a negligible inductive effect on enzyme biosynthesis by the experimental fungus. Moreover, the growth rate of A. flavipes was relatively varied with regard to the nature of the tested nitrogen source. The maximum growth rate was associated with l-asparagine (6.8 g/l) followed by ammonium oxalate (6.70 g/l), glutamine (6.05 g/l), and beef extract (5.7 g/l) as nitrogen sources. It could be concluded from these results that the induction of l-methioninase by A. flavipes is not only nitrogen regulated but also l-methionine dependent (inducible enzyme).
The l-methionine dependency of enzyme production by A. flavipes is similar to that reported for l-methioninase production by Aspergillus sp. RS-1a and Achromobacter starkeyi [39, 40], Pseudomonas ovalis [48], and Yarrwia lipolytica [6]. In contrast, l-methioninase biosynthesis by Pseudomonas putida [47] and Geotrichum candidum [7] were found to be l-methionine independent.
The effect of various l-methionine concentrations on enzyme productivity by A. flavipes was considered (Fig. 2). The initial concentration of fermentation medium l-methionine exerts a significant effect on the uptake of l-methionine and consequently on enzyme productivity by A. flavipes. The highest yield of l-methioninase (11.6 U/mg protein) and methionine uptake (94.0%) by A. flavipes was recorded using 0.8% l-methionine. Higher levels of l-methionine (3.2%) repress the enzyme yield by about 42.5% compared with the control. It could be concluded that the productivity of l-methioninase by the fungal isolate is l-methionine concentration dependent. Furthermore, the growth rate of A. flavipes was gradually increased with the level of l-methionine, reaching its highest value (6.0 g/l) at 0.8% l-methionine, followed by a gradual decrease to about 41.7% at 3.2% l-methionine. The lower enzyme yield with higher concentrations of l-methionine may be attributed to the downregulation of GATA gene transcription that hindered the gene expression of methioninase [10, 31], methionine catabolic repression, or the transinhibition phenomenon [35].
Effect of Different Phosphorus Sources on l-Methioninase Production
Phosphorus is one of the crucial elements controlling the integral structure, signal transduction, cellular reactions, and viability of microbial cells. The current data presented in Table 2 shows the significant effect of phosphorus sources on l-methioninase productivity and methionine uptake by A. flavipes. The maximum enzyme productivity (12.58 U/mg protein) and rate of methionine uptake (95.6%) by A. flavipes were obtained using 0.32% KH2PO4 as the sole phosphorus source, followed by 0.24% K2HPO4. In contrast, these parameters were relatively decreased with NaH2PO4 and Na2HPO4. However, a considerable reduction in A. flavipes biomass was determined using phosphorus free fermentation media, which increased by about 53.2% compared with the control. The preferential use of potassium dihydrogen phosphate could be attributed to the lower monovalent cations (K+) that may enhance cellular transport and membrane depolarization of fungal cells [22]. Similar results were obtained for l-methioninase production by Yarrowia lipolytica [6]. In partial connection with these results, both 0.1% KH2PO4 and 0.1% K2HPO4 (collectively) were used for maximum l-methioninase production in fermentation media of Aspergillus sp. RS-1a [40] and Pseudomonas ovalis TFO 3738 [48]. In contrast, Amarita et al. [2] showed that methioninase production by Brevibacterium linens was optimally recovered using 0.33% K2HPO4.
The Influence of Different Carbon Sources on l-Methioninase Production by A. flavipes
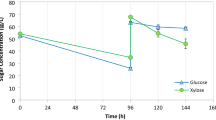
The use of different carbon sources as co-metabolic agents for l-methioninase production by A. flavipes was studied (Fig. 3). Among the different carbon sources, glucose was the optimum compound for l-methioninase production (12.58U/mg protein), uptake of methionine (95.6%), and growth of A. flavipes (6.0 g/l), followed by mannitol. Whereas a fairly stimulatory effect on the productivity of l-methioninase by the fungus was observed using other carbon sources, the lowest value was reached with citric acid (0.98 U/mg protein), cellulose (1.31 U/g protein), and potassium oxalate (1.37 U/mg protein). Interestingly, with the use of carbon-free medium, the three parameters of enzyme yield, methionine uptake, and biomass by A. flavipes were significantly reduced by about 97.4%, 68.61%, and 85%, respectively, compared with glucose-containing medium.
From these results, it can be deduced that in the absence of carbon compounds as co-dissimlators, A. flavipes seem to be metabolically grow on l-methionine. This may be due to its inability to metabolize the catabolized products of l-methionine such as α-keto-butyric acid and methanethiol. These results are supported by reports that glucose was the most favored co-dissimilating agent for l-methioninase production by filamentous fungi [41, 44, 49] including Acromobacter starkeyi [39] and Brevibacterium linens [2]. In contrast, Tanaka et al. [48] and Lockwood and Coombs [27] reported that glycerol and sucrose were the favored carbon sources for l-methioninase production by Pseudomonas ovalis and Trichomonas vaginalis, respectively.
Moreover, the influence of different concentrations of fermentation media glucose on the biosynthesis of l-methioninase by A. flavipes was investigated (Fig. 4). The results showed that the highest yield of l-methioninase (12.62 U/mg protein) and amino acid uptake (95.3%) by the experimental fungal isolate were detected using 1% glucose. Also, the l-methionine consumption profile clarified that the rate of amino acid uptake was reduced by about 68.5% using glucose-free medium compared with the optimum glucose level. Compared with the control, l-methioninase productivity and methionine uptake by A. flavipes were reduced about 9.6% and 8.8%, respectively, by 2% glucose-containing medium. The biomass was not correlated to the productivity of enzyme by A. flavipes. The maximum growth yield (8 g/l) of A. flavipes was obtained using 2% glucose.
It noteworthy that the induction and release of l-methioninase by A. flavipes is glucose regulated. This may be attributable to their role in the activation of plasma membrane H+-adenosine triphosphatase (ATPase) (i.e., cyclic adenosine monophosphate [cAMP] signaling and protein phosphorylation) [14] in contrast to the higher glucose concentration, which may interfere with the amino acid transports systems for l-methionine and thus block the inductive signals for the release of l-methioninase by the fungal cell. The optimum level of glucose for enzyme production by A. flavipes was similar to that reported for l-methioninase production by Aspergillus sp. RS-1a and Achromobacter sterkeyi [39, 40].
Effect of Initial pH of the Medium
The obtained data (Fig. 5) clearly show the dependence of enzyme production and amino acid uptake on the initial pH of the production medium. The optimum yield of l-methioninase (12.58 U/mg protein), the uptake of l-methionine (95.6%), and the biomass (6.0 ± 3.0 g/L) of A. flavipes were obtained at initial pH of 7.0. It is obvious from the results that the reduction of the three parameters is greater in the acidic medium (pH 2.0). The enzyme productivity, methionine uptake, and biomass yield of A. flavipes were decreased by 30%, 21.1% and 45%, respectively, comparing to the optimum pH value. Likewise, the methionine uptake profile showed a slight variation in the rate of amino acid uptake in the pH range of 5.0 to 7.0. The highest fungal productivity for l-methioninase at a neutral pH may be attributed to the balance of the ionic strength of plasma membrane, the maximum activity of H+-pumping ATPase, and the optimum fluxing of ions that influence the activities of calmodulin and adenylate cyclase [32]. Similar results were obtained for l-methioninase production by Aspergillus sp. RS-1a and Achromobacter sterkeyi [39, 41], Pseudemonas ovalis [48], and Brevibacterium linens [3].
Conclusion
This report focuses on screening for l-methioninase production from fungi isolated from Egyptian soil samples. A total of 21 fungal isolates were isolated as l-methionine decomposers, in which Aspergillus flavipes seems to be the most active fungal isolate for l-methioninase production. With regard to the biotechnological importance of l-methioninase, the selected fungal isolates were sustained for their enzyme yield by cultural optimization experiments to fulfill their enzyme productivity under submerged conditions. To the best of our knowledge, this is first report involving a screening system for l-methioninase production from soil fungi.
References
Abarca ML, Accensi F, Cano J, Cabanes FJ (2004) Taxonomy and significance of black aspergilli. Antonie Van Leewenhoek 86:33–49
Amarita F, Yvon M, Nardi M et al (2004) Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl Environ Microbiol 70:7348–7354
Arfi K, Landaud S, Bonnarme P (2006) Evidence for distinct l-methioninne catabolic pathways in the Yeast Geotrichum candidum and the bacterium Brevibacterium linens. Appl Environ Microbiol. 72:2155–2161
Bergstorm M, Ericson K, Hagenfeldt L (1987) PET study of methionine accumlation in glioma and normal brain tissue: competition with branched chain aminho acids. J Comput Assist Tomogr 11:208–213
Bilgrami KS, Verma RN (1981) Physiology of fungi, 2nd edn. Vikas Publishing, PVT, Ltd Indian, pp 23–27
Bondar DC, Beckerich JM, Bonnarme P (2005) Involvement of a branched-chain aminotransferase in production of volatile sulfur compounds. Yarrwialipolytica 71:4585–4591
Bonnarme P, Lapadatescu C, Yvon M, Spinnler HE (2001) l-methionine degradation potentialties of cheese-ripening microorganisms. J Dairy Res 68:663–674
Booth C (1971) The genus Fusarium. Commenwealth Mycological Institute, Kew
Breillout F, Antoine E, Poupon MF (1990) Methionine dependency of malignant tumors: a possible approach for therapy. J Natl Cancer Inst 82:1628–1632
Caddick MX, Peters D, Platt A (1994) Nitrogen regulation in fungi. Antoine van Leeuwenhoek 65:169–177
Challenger F, Charlton PT (1947) Studies on biological methylation. 10. The fission of the mono and disulfide links by molds. J Chem Soc (Lond) 424–429
Cuer A, Dauphin G, Kergomard A et al (1979) Flavour properties of some sulphur compounds isolated from cheeses. Lebensmittelwiss Technol 12:258–261
Domsch KH, Gams W, Anderson T (1980) Compendium of soil fungi. Academic Press
dos Passos JB, Vanhalewyn M, Brandao RL (1992) Glucose-induced activation of plasma membrane H+-ATPase in mutants of the yeast Saccharomyces cerevisiae affected in cAMP metabolism, cAMP-dependent protein phosphorylation, and the initiation of glycolysis. Biochemica et Biophysica Acta 1136:57–67
Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew
El Sayed ASA (2008) l-glutaminase production by Trichoderma koningii under solid state fermentation. Ind J Microbiol (in press)
Halpern BC, Clark BR, Hardy DN et al (1974) The effect of replacement of methionine by hemocystine on survival of malignant and normal adult mammalian cells in culture. Proc Natl Acad Sci USA 71:1133–1136
Hawkins DS, Park JR, Thomson BG et al (2004) Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated l-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res 10:5335–5341
Hess WC, Sullivan MX (1943) The cysteins, cystine and methionine content of proteins. J Biol Chem 151:635–642
Hori H, Takabayashi K, Orvis L et al (1996) Gene cloning and characterization of Pseudomonas putida l-methionine-α-deamino-γ-mercaptomethane-lyase. Cancer Res 56:2116–2122
Ito S, Nakamura T, Eguchi Y (1976) Purification and characterization of methioninase from P. putida. J Biohem 79:1263–1272
Jennings DH (1995) The physiology of fungal nutrition, 1st edn. Cambridge University Press, Cambridge
Johnson LF, Curl EA, Bond JH, Fribourg HA (1959) Methods for studying soil microflora—plant disease relationships. Burgess Publishing Co., MN
Kawashima K, Takeshima H, Higashi Y et al (1991) High efficacy of monomethoxypolyethylene glycol: conjugated l-asparaginase (PEG2-ASP) in two patients with hematological malignancies. Leukemia Res 15:525–530
Kokkinakis DM, Hoffman RM, Frankel EP (2001) Synergy between methionine stress and chemotheray in the treatment of brain tumor xenografts in athymic mice. Cancer Res 61:4017–4023
Kokkinakis DM, Schold SC Jr, Hori H, Nobori T (1997) Effect of long-term depletion of plasma methionine on the growth and survival of human brain xenografts in athymic mice. Nutr Cancer 29:195–204
Lockwood BC, Coombs GH (1999) Purification and characterization of methionine γ-lyase from Trichomonas vaginalis. Biochem J 279:675–682
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Lund F (1995) Differeniating Penicillium species by detection of indole metabolites using a filter paper mehod. Lett Appl Microbiol 20:228–231
Merricks DL, Salsbury RL (1974) Involvement of vitamin B6 in the dethiomethylation of methionine by rumen microorganisms. Appl Microbiol 28:106–111
Mitchell AP, Magasanik B (1984) Regulation of glutamate-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol Cell Biol 4:2758–2766
Monk BC, Niimi M, Shepherd MG (1993) The Candida albicans plasma membrane and H+-ATPase during yeast growth and germ tube formation. J Bacteriol 175:5566–5574
Nakayama T, Esaki N, Lee EJ et al (1984) Agric Biol Chem 48:2367–2369
Ohigashi K, Tsunetoshi A, Ichihara K (1951) The role of pyridoxal in methylmercaptan formation partial purification and resolution of methioninase. Med J Osaka Univ 2:111–117
Pall ML (1971) Amino acid transport in Neurospora crassa: IV. Properties and regulation of a methionine transport system. Biochem Biophys Acta 233:201–214
Pitt JI (1979) The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. Academic Press, London
Raper KB, Fennell DI (1965) The genus Aspergillus. The Williams and Wilkins Company, Baltimore
Rifai MA (1969) A revision of the genus Trichoderma. Commonwealth Mycological Institute, Kew
Ruiz-Herrera J, Starkey R (1970) Dissimilation of methionine by Achromobacter starkeyi. J Bacteriol 104:1286–1293
Ruiz-Herrera J, Starkey RL (1969) Dissimilation of methionine by fungi. J Bacteriol 94:544–551
Ruiz-Herrera J, Starkey RL (1969) Dissimilation of methionine by a demethiolase of Aspergillus species. J Bacteriol 99:764–770
Samson RA, Noonim P, Meijer M et al (2007) Diagnostic tools to identify black aspergilli. Stud Mycol 59:129–145
Snedecor GW, Cochran WG (1982) Statistical Methods, 6th ed. edn. Blackwell Science Ltd, London, p 147
Stahl WH, Ncqu B, Mandels GR et al (1949) Studies on the microbiological degradation of wool. 1. Sulfur metabolism. Arch Biochem 20:422–432
Tan Y, Sun X, Xu M et al (1998) Polyethylene glycol conjugation of recombinant methioninase for cancer therapy protein. Expr Purif 12:45–52
Tan Y, Xu M, Guo H et al (1996) Anticancer efficacy of methioninase in vivo. Anticancer Res 16:3931–3936
Tan Y, Xu M, Tan X et al (1997) Overexpression and large-scale production of recombinant l-methionine-α-deamine-δ-mercaptomethane-lyase for novel anticancer therapy. Protein Expr Purif 9:233–245
Tanaka H, Esaki N, Yamamoto T, Sodo K (1976) Purification and properties of methioninase from Pseudomonas ovalis. FEBS Lett 66:307–311
Tsugo T, Matsuko M (1962) The formation of volatile sulfur compounds during the ripening of the semisoft white mould cheese. In: Proceedings of the 16th international dairy congress, Copenhagen, Denmark, vol B, pp 385–394
Thompson JF, Morrison GR (1951) Determination of organic nitrogen: control of variables in the use of Nessler’s reagent. Anal Chem 23:1153–1157
Wiesendanger S, Nisman B (1953) La l-methionine demercapto desaminase: un novel enzyme a’ pyridoxal phosphate. Compt Rend 237:764–765
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalaf, S.A., El-Sayed , A.S.A. l-Methioninase Production by Filamentous Fungi: I-Screening and Optimization Under Submerged Conditions. Curr Microbiol 58, 219–226 (2009). https://doi.org/10.1007/s00284-008-9311-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9311-9