Abstract
A gene encoding the rice (Oryza sativa L.) 90-kDa heat shock protein (OsHsp90) was introduced into Escherichia coli using the pGEX-6p-3 expression vector with a glutathione-S-transferase (GST) tag to analyze the possible function of this protein under heat stress for the first time. We compared the survivability of E. coli (BL21) cells transformed with a recombinant plasmid containing GST-OsHsp90 fusion protein with control E. coli cells transformed with the plasmid containing GST and the wild type BL21 under heat shock after isopropyl β-d-thiogalactopyranoside induction. Cells expressing GST-OsHsp90 demonstrated thermotolerance at 42, 50, and 70°C, treatments that were more harmful to cells expressing GST and the wild type. Further studies were carried out to analyze the heat-induced characteristics of OsHsp90 at 42, 50, and 70°C in vitro. When cell lysates from E. coli transformants were heated at these heat stresses, expressed GST-OsHsp90 prevented the denaturation of bacterial proteins treated with 42°C heat shocks, and partially prevented that of proteins treated at 50 and 70°C; meanwhile, cells expressing GST-OsHsp90 withstood the duration at 50°C. These results indicate that OsHsp90 functioned as a chaperone, binding to a subset of substrates, and maintained E. coli growth well at high temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock protein 90 (Hsp90) is an abundant and evolutionarily conserved molecular chaperone in both eukaryotic and prokaryotic cells. Hsp90 is responsible for chaperone functions of maintaining structural integrity and regulating a subset of client proteins that are involved in cell cycle control and signal transduction, such as steroid-hormone receptors and kinases [9, 10].
Hsp90 was originally discovered as one of the proteins that increase in abundance upon heat stress [12]. It is now known that Hsp90 not only plays a key role in the cell response to stress, but also is widely distributed in organisms under normal conditions [7, 11]. Under stressful conditions, Hsp90 functions as a general chaperone to bind transiently to folding intermediates in vitro, preventing aggregation and supporting the refolding of intermediates to the native state [17]. Cells with low levels of Hsp90 or with Hsp90 mutants are hypersensitive to stress. Deleting Escherichia coli Hsp90 (HtpG) results in a slight growth disadvantage at elevated temperatures [1, 16]. Yeast Hsp90 (Hsc82 and Hsp82) is essential at any temperature [2, 4]. Several recent publications have also highlighted the importance of this chaperone complex in plant development [8].
To gain more insight into the protective role that Hsp90 plays in conditions under which unfolding and subsequent aggregation of polypeptides occur, Hsp90 isoforms were purified from bacteria, yeast, and plants in vivo or in vitro, and their characteristics and functions were studied [3, 5, 6]. In rice, however, there have been few studies on the characterization of Hsp90 isoforms.
Previously, we isolated the gene encoding rice Hsp90 (OsHsp90), which was predicted to localize in the endoplasmic reticulum. The gene was found in response to several abiotic stresses including high temperature (42 and 50°C) [7]. To further analyze the characteristics of OsHsp90, in the present study we introduced a cDNA clone encoding rice OsHsp90 into the pGEX-6p-3 vector for expression and purification of a glutathione-S-transferase (GST) fusion protein in E. coli and examined the heat tolerance of bacterial cells expressing OsHsp90 in vivo and in vitro.
Materials and Methods
Materials
E. coli Jm109 and BL21, used as expression host strains, were cultured aerobically in Luria-Bertani medium and 2× YT medium (1% yeast extract, 1.6% tryptone, and 0.5% NaCl), respectively. The vector used in this study was pGEX-6P-3 (Amersham Pharmacia), which contains a tac promoter for chemically inducible, high-level GST fusion protein expression.
Construction of Expression Plasmid
Isolation of the rice OsHsp90 gene (GenBank accession no. AB037681) has been described previously [7]. Specific primers designed for OsHsp90 were as follows: sense, 5′-CACGGGATCCACGATGCGCAAGTGGGCGCTCTC-3′; and antisense, 5′-GGACGCGGCCGCCTACAGCTCGTCCTTAT-3′. We introduced BamHI and NotI sites as linkers to pGEX-6P-3, indicated in italics in the nucleotide sequences. The PCR product was ligated into pGEX-6p-3 and digested with appropriate restriction enzymes to construct the plasmid pGEX-6p-3-OsHsp90.
Expression and Purification of OsHsp90 Protein in E. coli
BL21 cells transfected with pGEX-6P-3-OsHsp90 were used for expression of GST-OsHsp90. The cells were cultured at 37°C overnight in 2× YT medium supplemented with 100 mg L−1 ampicillin. The cultures were then inoculated into fresh prewarmed 2× YT medium (1:100 dilution) containing 100 mg L−1 ampicillin and incubated at 37°C with gentle shaking. When the optical density at 600 nm (OD600) reached 0.5, expression of the fusion protein was induced by adding isopropyl-β-d-thiogalactoside (IPTG) to a final concentration of 1 mM for up to 2 h. Cell pellets were resuspended in precooled lysis buffer (100 mM NaCl, 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 2% Triton X-100) supplemented with 1 mM phenylmethylsulfonyl fluoride, 5 mM dithiothreitol (DTT), and 2 mg mL−1 lysozyme. The suspension was incubated on ice for 30 min, then centrifuged at 15,000 g for 30 min. The resulting supernatant was loaded onto a glutathione Sepharose 4B column (Amersham Pharmacia) pre-equilibrated with phosphate-buffered saline (PBS). Nonspecifically bound proteins were removed by washing with PBS supplemented with 0.5% Triton X-100. Then the column was washed with 2 bed vol of sonication buffer (50 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA, 1 mM DTT). The fusion protein GST-OsHsp90 was eluted with 100 mM Tris-HCl (pH 8.0) buffer containing 20 mM reduced glutathione, then objected to SDS/PAGE.
Dot Assay of Recombinant E. coli Under Heat Shock Stress
E. coli cultures were incubated at 37°C until cells reached the midlog phase (OD600 = 0.5) before induction with 1 mM IPTG for an additional 2 h or not. The concentration of these cultures was identified to OD600 = 0.5 and then diluted serially (1:10, 1:100, and 1:1000, respectively). Ten microliters of each sample was spotted onto the 2× YT plates with or without 1 mM IPTG induction, and then the plates were subjected to 42, 50, and 70°C heat shock treatments for 30 min, respectively, before incubation overnight at 37°C.
Heat-Induced Characteristics of OsHsp90
For the thermostability assay, cells were grown as above, and lysate proteins were extracted from E. coli cells in precooled lysis buffer. Samples were then incubated at 0, 42, 50, or 70°C for 20 min and centrifuged at 17,000 g for 10 min at 4°C.
For the growth curve assay under heat stress, the flasks were transferred to a water bath operating at 50°C for the duration of the experiment.
Statistical Analysis
All experiments were carried out in triplicate, and three samples in each individual determination were examined each time. The values shown in the figures are mean values ± SD.
Results
Expression of OsHsp90 Recombinant Protein in E. coli
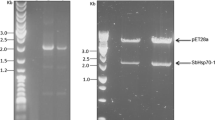
Rice OsHsp90 gene was introduced into E. coli to analyze the possible function of this protein under heat stress. Escherichia coli BL21, transformed with pGEX-6p-3-OsHsp90 and induced with IPTG, produced a protein with an approximate molecular mass of 118.8 kDa (GST-OsHsp90 fusion protein), which was absent in noninduced cells (Fig. 1a). Along with the increasing induction time, the expression level of GST-OsHsp90 fusion protein increased, and the most prominent protein was found in expression lysates after 120 min of induction (Fig. 1a). The GST-OsHsp90 fusion protein was purified by affinity chromatography on a glutathione Sepharose 4B column, had a molecular weight of 118.8 kDa, and contained a 26-kDa GST tag and 92.8-kDa OsHsp90 (Fig. 1b). Under normal conditions (37°C), there were no differences in growth rate among E. coli cells transformed with GST-OsHsp90, GST, and the wild type (BL21), whether or not there was IPTG induction (data not shown).
Expression and purification of GST-OsHsp90 fusion protein. a E. coli cells were cultured at 37°C with additional 1 mM IPTG induction for up to 120 min, and total crude extract was analyzed by SDS-PAGE. b GST-OsHsp90 fusion protein (lane 3) was purified from E. coli lysates after 0 min (lane 1) and 120 min (lane 2) of induction. M, molecular mass markers. Arrows indicate GST-OsHsp90
Thermotolerance of E. coli Cells Expressing the GST-OsHsp90 Fusion Protein
To determine whether OsHsp90 could enhance the thermotolerance of E. coli, the effects of heat stress on growth of E. coli cells transformed with GST-OsHsp90, GST, and the wild type (BL21) were examined. Diluted aliquot cultures of OD600 = 0.5 were spread on 2× YT plates treated with different heat shock stresses. As shown in Fig. 2, there were no obvious differences in growth among the three kinds of E. coli cells not receiving any heat shock, whether or not IPTG induction was carried out. However, when cells without IPTG induction underwent 42, 50, and 70°C heat shock stresses, all of them were damaged, and almost no cells were observed on the plates under 70°C stress. However, IPTG induction provided a greater advantage to the growth of E. coli-producing GST-OsHsp90 than to the growth of the other two cells under heat stress, especially at 50 and 70°C. All these results showed that the expressed OsHsp90 recombinant protein conferred thermotolerance to the host cells.
Dot assay of E. coli cells expressing GST-OsHsp90, GST, and the wild type (BL21). IPTG was added to the cultures to induce recombinants expressing aimed protein. Ten microliters of the serially diluted bacterial suspension was spotted onto 2× YT plates, and then they were exposed to heat shock for 30 min before recovery incubation at 37°C
Proteins from E. coli Cells Producing GST-OsHsp90 Show Higher Thermostability
To understand whether OsHsp90 can protect E. coli proteins from precipitation during heat stress, we analyzed the stability of E. coli proteins isolated from cells expressing GST-OsHsp90 following a heat treatment at 42, 50, and 70°C. As shown in Figs. 3a and b, most lysate proteins in E. coli transformed with GST-OsHsp90 without IPTG induction precipitated on exposure to 42°C for 20 min. However, the precipitation of such proteins was prevented as sufficient GST-OsHsp90 was present. At 50 and 70°C, larger amounts of lysate proteins precipitated in the absence of GST-OsHsp90. In contrast, most of the lysate proteins remained in the supernatant when the fusion protein was present. Furthermore, we studied the effect of duration of exposure to 50°C on such binding characterization. As incubation time increased, the ability of the fusion protein to prevent the precipitation of other proteins decreased, and upon exposure to 50°C for 50 min, the fusion protein completely lost its substrate binding characteristic and precipitated with the other proteins (Fig. 3c).
Heat-induced characteristics of OsHsp90 in vitro and in vivo. a, b Prevention of heat-induced precipitation of bacterial lysate proteins in the presence of GST-OsHsp90 at 0, 42, 50, and 70°C for 20 min. Lysate was prepared from bacteria in which OsHsp90 had been induced with (+) or without (–) IPTG. c Lysate samples were subjected to 50°C for up to 50 min with IPTG induction. The resulting supernatant (S) and precipitating (P) fractions were subjected to SDS-PAGE. C, purified GST-OsHsp90 fusion protein. d Growth curves for E. coli subjected to heat shock at 50°C; BL21 cells used as a negative control (△); cells expressing GST with (◆) or without (◇) IPTG induction; and cells expressing GST-OsHsp90 with (■) or without (□) IPTG induction. The three curves of BL21 cells, cells expressing GST and GST-OsHsp90 without IPTG induction nearly overlapped with each other
Then we determined the growth rate of BL21 cells expressing GST-OsHsp90 by induction with IPTG under 50°C. Cells transformed with GST and wild-type (BL21) cells were used as controls. The prolonged heat shock seriously affected the growth rate of wild-type cells and cells transformed with GST or GST-OsHsp90 without IPTG induction. On the contrary, although there was little enhancement of cell expression of GST, expression of GST-OsHsp90 made the growth rate of E. coli increase more obviously (Fig. 3d).
Discussion
Under heat stress conditions, dysfunction of enzymes and proteins usually occurs. Therefore, maintaining proteins in their functional conformations and preventing aggregation of nonnative proteins are particularly important for cell survival under stress. Many stress-responsive proteins, especially Hsp90 s, have been shown to act as molecular chaperones, which function in the stabilization of proteins and membranes and in the assistance of protein refolding under stress conditions [13].
Hsp90 s are conserved in all living organisms and are especially widely distributed in plants. Expression of Hsp90 was mostly related to high-temperature stress. In eukaryotes, the ubiquitous and abundant members of the Hsp90 chaperone family facilitate the folding and conformational changes of a broad array of proteins important in cell signaling, proliferation, and survival [4, 10]. In prokaryotes, the Hsp90 analogue, HtpG, which demonstrates 40% similarity to eukaryotic Hsp90, is essential for thermotolerance in cyanobacteria [15].
To study the function of Hsp90 in vivo, we introduced the rice OsHsp90 gene into the organism E. coli (BL21). In GST-OsHsp90-transformed cells, production of recombinant OsHsp90 allowed E. coli to survive at temperatures of 42, 50, and 70°C, which were more harmful or even lethal to cells expressing GST and the wild type. In E. coli, a similar function to prevent protein denaturation has also been shown for DnaK, DnaJ, and GroE proteins [14].
Meanwhile, to determine whether the expression of OsHsp90 recombinant protein enhances the survival of E. coli, we first tested the substrate binding characteristics of OsHsp90 under different heat shock conditions. It was found that OsHsp90 effectively prevented the denaturation of lysate proteins of E. coli cells at elevated temperatures to different degrees. Furthermore, we found that cells expressing OsHsp90 could withstand 50°C heat stress treatment, with both BL21 and cells harboring GST, with or without IPTG induction, serving as controls. It can be speculated that such substrate binding characteristics of OsHsp90 resulted in an enhancement of the tolerance of E. coli cells to heat stress. The results also reveal that OsHsp90 assisted a wide range of substrates of E. coli to a certain extent, and severe stress damage also caused loss of activity of the recombinant protein. In any case, OsHsp90 played an important role when bacterial cells were exposed to heat shock conditions, and overproduction of this foreign protein directly contributed to thermotolerance and thermoprotection of the host cells.
Herein, our results showing enhancement of thermotolerance of recombinant bacteria cells indicate that expression of OsHsp90 in host cells confers a protective function against damage of proteins. OsHsp90 buffered many bacterial proteins from precipitation under different heat shock conditions and, thus, maintained the growth of E. coli cells expressing OsHsp90 upon exposure to 50°C. It is concluded that OsHsp90 appears to be a dedicated chaperone for a defined set of substrates under normal and stressful conditions, and functions in E. coli under heat shock conditions. Based on our results, it is reasonable to speculate that the protective mechanism of OsHsp90 might be similar in prokaryotes and eukaryotes under environmental stress conditions, and it is expected that strongly heat-, salt-, desiccation-, and other stress-tolerant plant varieties, by overexpression of OsHsp90, will be developed for practical use. Furthermore, to clarify the mechanism of such stress tolerance in plants, it will be important to determine exactly the functional proteins interacting with OsHsp90 and establish connections between these master proteins and the expression of stress tolerance.
References
Bardwell JCA, Craig EA (1988) Ancient heat shock gene is dispensable. J Bacteriol 7:2977–2983
Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S (1989) Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol 9:3919–3930
Chu LF, Lee WC, Yang PC, Chu R, Huang TY, Mao SJ (1997) One-step HPLC purification procedure for porcine brain 90-kDa heat shock protein. Protein Expr Purif 10:180–184
Kimura Y, Matsumoto S, Yahara I (1994) Temperature-sensitive mutants of hsp82 of the budding yeast Saccharomyces cerevisiae. Mol Gen Genet 242:517–527
Krishna P, Reddy RK, Sacco M, Frappier RH, Felsheim RF (1997) Analysis of the native forms of the 90 kDa heat shock protein (hsp90) in plant cytosolic extracts. Plant Mol Biol 33:457–466
Larreta R, Soto M, Alonso C, Requena JM (2000) Leishmania infantum: gene cloning of the GRP94 homologue, its expression as recombinant protein, and analysis of antigencity. Exp Parasitol 96:108–115
Liu D, Zhang X, Cheng Y, Takano T, Liu S (2006) rHsp90 gene expression in response to several environmental stresses in rice (Oryza sativa L.). Plant Physiol Biochem 44:380–386
Marrs KAE, Casey S, Capitant SA, Bouchard RA, Dietrich PS, Mettler IJ, Sinibaldi R (1993) Characterization of two maize hsp90 heat shock protein genes and expression during heat shock, embryogenesis, and pollen development. Dev Genet 14:27–41
Park M, Kang CY, Krishna P (1998) Brassica napus hsp90 can autophosphorylate and phosphorylate other protein substrates. Mol Cell Biol 185:33–38
Pratt WB (1997) The role of Hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol 37:297–326
Reddy RK, Chaudhary S, Patil P, Krishna P (1998) The 90 kDa heat shock protein (hsp90) is expressed throughout Brassica napus seed development and germination. Plant Sci 131:131–137
Sanchez ER, Housley PR, Pratt WB (1986) The molybate-stabilized glucocorticoid binding complex of L-cells contains a 98–100 Kdalton steroid binding phosphoprotein and a 90 Kdalton nonsteroid-binding phosphoprotein that is part of the murine heat-shock complex. J Steroid Biochem 24:9–18
Schöffl F, Prändl R, Reindl A (1998) Regulation of the heat-shock response. Plant Physiol 117:1135–1141
Schroder H, Langer T, Hartl FU, Bukau B (1993) DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J 12:4137–4144
Tanaka N, Nakamoto H (1999) HtpG is essential for the thermal stress management in cyanobacteria. FEBS Lett 458:117–123
Thomas JG, Baneyx F (1998) Roles of the Escherichia coli small heat shock proteins IbpA and IbpB in thermal stress management: comparison with CIpA, CIpB, and HtpG in vivo. J Bacteriol 180:5165–5172
Wiech H, Buchner J, Zimmermann R, Jakob U (1992) Hsp90 chaperones protein folding in vitro. Nature 358:169–170
Acknowledgments
This work was supported by the Excellent Teachers Program Foundation of Heilongjiang University (QL200729) and the Heilongjiang Province Postdoctoral Science Foundation (LBH-Z07029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, D., Lu, Z., Mao, Z. et al. Enhanced Thermotolerance of E. coli by Expressed OsHsp90 from Rice (Oryza sativa L.). Curr Microbiol 58, 129–133 (2009). https://doi.org/10.1007/s00284-008-9288-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9288-4