Abstract
The success of rhizobial inoculation on plant roots is often limited by several factors, including environmental conditions, the number of infective cells applied, the presence of competing indigenous (native) rhizobia, and the inoculation method. Many approaches have been taken to solve the problem of inoculant competition by naturalized populations of compatible rhizobia present in soil, but so far without a satisfactory solution. We used antibiotic resistance and molecular profiles as tools to find a reliable and accurate method for competitiveness assay between introduced Bradyrhizobium sp. strains and indigenous rhizobia strains that nodulate peanut in Argentina. The positional advantage of rhizobia soil population for nodulation was assessed using a laboratory model in which a rhizobial population is established in sterile vermiculite. We observed an increase in nodule number per plant and nodule occupancy for strains established in vermiculite. In field experiments, only 9% of total nodules were formed by bacteria inoculated by direct coating of seed, whereas 78% of nodules were formed by bacteria inoculated in the furrow at seeding. In each case, the other nodules were formed by indigenous strains or by both strains (inoculated and indigenous). These findings indicate a positional advantage of native rhizobia or in-furrow inoculated rhizobia for nodulation in peanut.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizobia are symbiotic bacteria that elicit formation of nodules on the roots of legume hosts, within which the bacteria fix atmospheric nitrogen into ammonia. A fully functional symbiosis requires successful completion of numerous steps, beginning with the exchange of recognition signals between the plant and bacteria. The signaling processes is started by the plant that releases root exudates, including flavonoids and nutrients. Rhizobia are chemotactic toward them and respond to a characteristic plant flavonoid composition by initiating synthesis of specific lipochitooligosaccharide (LCO). These compounds, which are known as nod factors, trigger the early stages of nodule development, including root hair deformation, cortical cell division, and nodule morphogenesis [10].
Peanut (Arachis hypogaea L.), a member of the Leguminosae family, is usually nodulated by the bacterial microsymbiont Bradyrhizobium sp. [23]. Recent studies demonstrated that peanut is nodulated by both slow and fast rhizobia [20]. Peanut rhizobia initiate infection at the bases of root hairs in the axils of emerging lateral roots through spaces between epidermal cells, and subsequently proliferate in intercellular spaces before invading the cortical cells, a process termed crack entry. After entry, rhizobia cells occupy the space between epidermal and cortical cells, spread further through the root cortex via an intercellular matrix [4], and are eventually released into the plant cells. The resulting symbiosomes contain bacteriods; this is the state at which rhizobia fix nitrogen [18].
Biological nitrogen fixation (BNF) is an important and well-studied process in plant nutrition. International interest in environmentally sustainable development using renewable resources has drawn attention to the important ecological role of BNF in supplying nitrogen for agriculture [17]. A problem of great economic importance in microbial ecology concerns the efficiency of nitrogen-fixing bacteria to develop nodules on legumes such as peanut, soybean, and bean. The aim of inoculation is to provide sufficient numbers of viable (effective) rhizobia to induce a rapid colonization of the rhizosphere [8]. The most common method of inoculation involves treating the seed with a peat-based powder or liquid inoculant before planting [9]. Some strains of rhizobia effectively increase symbiotic nitrogen fixation under controlled experimental conditions. However, several attempts to use such strains to improve nitrogen fixation under agricultural conditions failed. The success of inoculation is limited by several factors, including the presence of competing indigenous rhizobia, which presents a “competition barrier” against nodulation by the inoculum strain [21].
Inoculation of peanut is not a common practice in the province of Córdoba (Argentina) because soils usually contain an indigenous population of rhizobia capable of nodulating an introduced peanut crop [3]. As a result, a positive response to inoculation may not be observed under field conditions. In a one-year study, seed inoculation treatment with Bradyrhizobium sp. had no effect on peanut seed yield [7]. On the other hand, another study showed that pod yield was higher when inoculant was applied in the furrow than when applied to peanut seeds before planting [12]. Similarly, in field experiments in various soils of Argentina, nodule number was higher for in-furrow inoculation than for seed inoculation [3]. These results suggest that in-furrow inoculation of an improved strain may be an advantage to it when competing with native rhizobia.
The contribution of bacterial distribution to the success of an introduced strain to out-compete natural populations in inducing nitrogen-fixing nodules in peanut needs to be studied further in order to design an inoculation technique that can overcome the competition problem. The purpose of this study was to evaluate the effect of bacterial inoculation techniques on the ability of selected rhizobia to nodulate peanut in soils containing an indigenous population of rhizobia. To do that, we developed a lab model. This is the first reported field experiment to assess competitiveness for nodule occupancy on peanut roots. Consequently, the results are useful in explaining the competitive behavior of an inoculant strain.
Materials and Methods
Bacterial Strains and Growth Conditions
For the control we used Bradyrhizobium sp. C-145, which is the strain recommended by INTA (Instituto Nacional de Tecnología Agropecuaria) for inoculant production in Argentina. A collection of native strains of bradyrhizobia were evaluated with respect to their intrinsic antibiotic resistance characteristics and molecular profile. Each strain was grown in YEM medium [25] for 4–6 days at 28°C. YEM broth cultures of C-145 strain were harvested at the late exponential phase of growth (109 cells mL−1), and used in laboratory assays to inoculate peanut seeds and sterile vermiculite pots as described below.
Isolation of Native Bacteria
Native rhizobia were isolated from root nodules of peanuts grown in untreated soil from La Aguada (Córdoba, Argentina). Nodules were surface sterilized for 4–5 min with a mercuric chloride solution (0.1% w/v) and then rinsed five times with sterile distilled water. Each nodule was stabbed with a sterile toothpick in 200 μL saline solution (0.9% NaCl), and the contents were spotted on YEM agar plates. The plates were incubated for 6–8 days at 28°C [19]. To confirm their ability to nodulate A. hypogaea L., isolates were reinoculated onto plants growing in sterilized vermiculite (nodulation assay). A negative control (noninoculated seedling) and positive control (inoculated with a strain recommended as a peanut inoculant) were included.
Generation of Antibiotic-resistant Mutants
The wild-type C-145 strain was grown in YEM broth and agar with increasing concentrations of gentamicin or streptomycin (selective pressure). Mutants resistant to gentamicin at 80 μg mL−1 (termed C-145 GmR) and to streptomycin at 100 μg mL−1 (termed C-145 StrR) were selected. The nodulating and nitrogen-fixing capacity of C-145 and the mutants was evaluated by determining nodule number, nodule dry weight, percent effective nodules, and plant dry weight using standard nodulation assays in a greenhouse.
Laboratory Assays
Mutant strains C-145 GmR and C-145 StrR, described above, were used to assess the effect of bacterial position in a competition assay. Vermiculite-filled pots were inoculated with a rhizobial suspension made by diluting a YEM log-phase culture with sterile nitrogen-free Hoagland solution to a cell concentration of 105 cells/g or 4.6 × 106 cells/pot. Pots were placed in a chamber at 28/24°C and watered with nitrogen-free Hoagland solution. Bacterial numbers grew up to 106 cells/g of vermiculite by 2 weeks and remained constant thereafter. Based on this result, inoculated and noninoculated peanut seeds were sown in pots 15 days after establishment of strains in vermiculite. Peanut seeds were surface sterilized and inoculated (5 × 106 cells/seed) with either the C-145 GmR or the C-145 StrR strain. Seeds inoculated with C-145 GmR were sown in pots filled with vermiculite containing an established C-145 StrR or vice versa (“cross inoculation”). Plants were grown in a chamber at 28/24°C under a 16/8-h light/dark regime, supplied with nitrogen-free Hoagland solution as needed [13], and harvested 30 days after planting. Competitiveness was assessed by identifying the bacteria inside 100 excised nodules, and nodule occupancy was examined by growing the bacteria on plates containing different antibiotics. For the nodulation assay, plants were grown as above, and nodules were picked and counted 30 days after planting. All experiments were performed in triplicate.
ERIC-PCR Analysis
Nodule occupancy and diversity of peanut rhizobial populations were determined using the enterobacterial repetitive intergenic consensus (ERIC)-PCR method. The template used for ERIC-PCR was genomic DNA obtained from isolated bacteria. Amplifications were performed with primers designed originally by Versalovic et al. [24] (ERIC1: 5′-ATGTAAGCTCCTGGGGATTCAC-3′; ERIC2: 5′-AAGTAAGTGACTGGGGTGAGCG-3′). PCR reactions were carried out essentially as described by de Bruijn [11] for rhizobia and other soil bacteria.
Field Experiments
Trials were conducted at La Aguada (Córdoba, Argentina). Strain C-145 GmR was used for seed inoculation and in-furrow inoculation with noninoculated seeds as a control. Seeds were planted in completely randomized block design with four replicates. A runner Virginia-type peanut (Manigran) cultivar was hand-planted in rows 0.7 m apart with 16 seeds/m. Before sowing, seeds were inoculated (200 mL/50 kg seed) with a commercial aqueous inoculant from Síntesis Química SAIC, containing 109 cells/mL (concentration checked by plating on YEM agar medium). Each inoculated seed contained approximately 2 × 106 cells. For in-furrow inoculation, the inoculant was applied directly on the seedbed (1.5 L/ha). At growth stage R1 (flowering, 31 days after planting) [5], plants were harvested, the root systems were washed, and nodules were picked and counted. To determine effectiveness, 100 nodules for each replication were cut in halves and the percentage of effective nodules was calculated based on the presence of leghemoglobin (assessed by a strong pink color; a nodule with weak pink color or white color was considered ineffective). Plant shoots were dried (70°C, 72 h) and weighed. Nodule occupancy was evaluated as above in YEM agar medium with or without gentamicin. An antibiotic disk test was used to evaluate co-occupancy when necessary.
Statistical Analysis
Experiments were conducted using a completely randomized design. Values presented were means of repeated experiments. Data were subjected to one-way analysis of variance, followed by a comparison of multiple treatment levels with the control using post hoc Fisher’s LSD (least significant difference) test with p ≤ 0.05 considered significant. Statistical analyses were performed with Infostat software version 1.0 (Group Infostat, Universidad Nacional de Córdoba, Argentina).
Results
We previously reported that the lack of response to peanut inoculation in soils of Argentina may be due to the presence of a competitive indigenous population of rhizobia [3]. Therefore, we decided to type by means of ERIC-PCR control strain C-145 and rhizobial strains isolated from the soils. The wild-type C-145 strain and indigenous isolates each exhibited a unique profile (Fig. 1), indicating that peanut-nodulating rhizobia are highly diverse.
ERIC-PCR fingerprint patterns of bradyrhizobial isolates. ERIC-PCR products shown were generated by using chromosomal DNA of the following Bradyrhizobium sp. strains or isolates: C-145 (lanes 1 and 18), 11LA (lane 2), 18LA (lane 3), 26LA (lane 4), 27LA (lane 5), 29LA (lane 6), 33LA (lane 7), 37LA (lane 8), 4AG (lane 9), 5AG (lane 10), 10AG (lane 11), 13AG (lane 12), 15AG (lane 13), 20AG (lane 14), 45AG (lane 15), 55AG (lane 16), 62AG (lane 17). Lane S shows DNA size markers (GeneRuler 100 bp, DNA Ladders 80-1000 bp, Fermentas)
To assess the influence of well-established populations in the soil profile, which presumably interfere with nodulation by an introduced strain, we developed an experimental laboratory model in which a rhizobial population was established in sterile vermiculite (Fig. 2). Mutants of a C-145 strain resistant to gentamicin at 80 μg mL−1 and to streptomycin at 100 μg mL−1 (termed C-145 GmR and C-145 StrR, respectively) were used to evaluate the effect of rhizobial distribution. Symbiotic properties were the same for each of the three strains (wild-type C-145 and both mutants), suggesting that the induction of antibiotic resistance had no effect on symbiosis. We observed an increased percentage of nodule occupancy for the strain established in vermiculite relative to the strain inoculated on seed (Fig. 3). Moreover, nodule number per plant was higher when inoculated or noninoculated seeds were placed in pots containing an established strain in vermiculite compared to inoculated seeds placed in vermiculite-free rhizobia (Fig. 4).
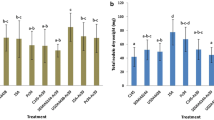
Effect of different treatments on nodule number based on the laboratory model shown in Fig. 2. StrSeed, GmSeed: seed inoculation of C-145 StrR and C-145 GmR strains. StrVerm, GmVerm: C-145 StrR and C-145 GmR strains established in vermiculite. Values presented are means of three repeated experiments. Different letters (a, b) indicate significant difference (p < 0.05) between treatments by Fisher’s LSD test
Based on these results, we did one field experiment in which the strain C-145 GmR was inoculated in different ways so that distribution of strains was altered. This mutant provides a useful tool to evaluate nodule occupancy in soil environments since we confirmed that native strains are sensitive to gentamicin. Significant differences were observed in nodule number, symbiotic effectiveness, and plant dry biomass for in-furrow inoculation compared to seed inoculation or control treatments (Fig. 5). Nodule occupancy of the C-145 GmR strain was very high for in-furrow inoculation (Fig. 6), indicating a positional advantage of in-furrow inoculated rhizobia for nodulation in peanut.
Effect of peanut inoculation with the C-145 strain on plant nodulation (nodule number/plant and percent effective nodules) and growth (shoot dry weight) in field experiments. Bars are means of four replicates for in-furrow inoculation (light gray bars), seed inoculation (dark gray bars), and control without inoculation (black bars). * indicates significant difference (p < 0.05) between treatments by Fisher’s LSD test
Nodule occupancy in field experiments using in-furrow inoculation, seed inoculation, or no inoculation (control). Bars are means of four repeated experiments for nodule occupancy by C-145 GmR strain (light gray bars), native strains (dark gray bars), and both strains (black bars). * indicates significant difference between strains by Fisher’s LSD test
Discussion
Peanut is an economically important legume used for direct consumption as well as for manufacturing numerous food products. Argentina is one of the major peanut producers in the world and about 94% of its crop is produced in the province of Córdoba. Inoculation of peanut with nitrogen-fixing bacteria is not a common practice in this country. Peanut is considered a “promiscuous” species that is nodulated by rhizobia that also nodulate a variety of other legumes [2]. Rhizobia capable of nodulating peanut may be inefficient in fixing nitrogen but the strains used as inoculants must not only be efficient at nitrogen fixing but also an outstanding competititor against rhizobia soil native populations.
The response of peanut to inoculation with bradyrhizobia is affected by many factors, but few studies have addressed the effect of indigenous rhizobia populations. In our experiments, while few nodules (∼9%) were formed solely by the seed-inoculated strain, 91% of them contained either indigenous strains alone or in combination with the introduced strain C-145 mutant. However, in-furrow inoculation resulted in a much higher nodulation percentage (∼78%) of nodules occupied by the inoculated strain (Fig. 6), probably the result of a positional effect, while the strain inoculated in-furrow was able to reach and infect emergent roots. Rhizobia inoculated on the seed remain concentrated around the seed coat [26]. These rhizobia are then taken away from the growing tip root; therefore, they have to move in the soil, attach to the growing root, and move along the root to reach the infection sites. Inoculated rhizobia have very limited vertical mobility and may therefore be hindered from reaching infectible zones of the roots beyond a certain soil depth [16]. Our previous experiments showed that noninoculated peanuts were fairly well nodulated, most probably by indigenous rhizobia characterized as Bradyrhizobium sp. strains [3], indicating that soils at Rio Cuarto frequently contain an indigenous rhizobia population capable of nodulating an introduced peanut crop, although their effectiveness in nitrogen fixation is unclear.
The efficiency of inoculation depends on the number of viable rhizobia available close to the root tip of legumes, which is controlled by several factors. Direct inoculation of the seed bed at the time of sowing (in-furrow inoculation) reduces not only the damage of manipulation of seeds with fragile seed coats, but it also reduces the adverse effect that pesticides and/or fungicides on the seed coat have on rhizobia. In addition, it also reduces the loss of viable bacteria through seed-drilling equipment or by lifting of the seed coat out of the ground during germination [6]. Therefore, these results and those of a previous study showed that in-furrow inoculation increased nodulation in peanut [3].
Seed inoculation and in-furrow inoculation techniques each have advantages and disadvantages. Selection of one of these methods depends on availability of equipment, seed size, fragility of cotyledons, presence of fungicides, and convenience. In this study, both antibiotic-resistant mutants and ERIC-PCR typing were used to evaluate nodule occupancy after seed or in-furrow inoculation of peanut grown in soils with indigenous rhizobial populations. Abaidoo et al. [1], while studying a bradyrhizobial isolate that nodulates soybean, confirmed that ERIC was an efficient typing technique. Differences in antibiotic-resistance profiles and in electrophoretic patterns produced with ERIC-PCR primers revealed the high diversity of peanut-nodulating rhizobia that inhabit the soils of the peanut-producing area. These findings are consistent with previous reports suggesting that soils contain a high genetic diversity and heterogeneity (often termed “promiscuity”) of rhizobial strains that nodulate peanut [22, 23, 27]. Furthermore, recent studies demonstrated the promiscuity of both slow and fast rhizobia in inducing nodules on peanuts [15, 20].
Our results show that in-furrow inoculation with the C-145 strain enhanced nodulation in field experiments at La Aguada. Competitiveness assays for nodule occupancy indicated that introduced strains can compete with indigenous strains provided that they are inoculated in-furrow. The importance of the effect of bacterial positioning is shown in nodulation of soybean, a legume infected by rhizobia through an infection thread [14]. We show here a similar effect was found in peanut, a legume infected by rhizobia in the different way, crack entry. Therefore, the importance of bacterial positioning in the soil for nodule formation appears to be a general phenomenon and does not depend on the route of infection.
In conclusion, the present findings indicate that by placing bradyrhizobia in the seed bed (furrow), the chances of inoculated bacteria to encounter breaks in secondary root epidermis are improved and their chances for establishing symbiosis are therefore increased. In-furrow inoculation is recommended for peanut or other legume crops when indigenous rhizobial populations are present in the soil.
References
Abaidoo RC, Keyser HH, Singleton PW, Borthakur D (2002) Comparison of molecular and antibiotic resistance profile methods for the population analysis of Bradyrhizobium spp. (TGx) isolates that nodulate the new TGx soybean cultivars in Africa. J Appl Microbiol 92:109–117
Alwi N, Wynne JC, Rawlings JO, Schneeweis TJ, Elkan GE (1989) Symbiotic relationship between Bradyrhizobium strains and peanut. Crop Sci 29:50–54
Bogino P, Banchio E, Rinaudi L, Cerioni G, Bonfiglio C, Giordano W (2006) Peanut (Arachis hypogaea) response to inoculation with Bradyrhizobium sp. in soils of Argentina. Ann Appl Biol 148:207–212
Boogerd FC, van Rossum D (1997) Nodulation of groundnut by Bradyrhizobium: a simple infection process by crack entry. FEMS Microbiol Rev 21:5–27
Boote KJ (1982) Growth stages of peanut (Arachis hypogaea L.). Peanut Sci 9:35–40
Brockwell J, Gault RR, Herridge DF, Morthorpe LJ, Roughley RJ (1988) Studies on alternative means of legume inoculation: microbiological and agronomic appraisals of commercial procedures for inoculating soybeans with Bradyrhizobium japonicum. Aust J Agric Res 39:965–972
Castro S, Permigiani M, Vinocur M, Fabra A (1999) Nodulation in peanut (Arachis hypogaea L.) roots in the presence of native and inoculated rhizobia strains. Appl Soil Ecol 13:39–44
Catroux G, Hartmann A, Revellin C (2001) Trends in rhizobial inoculant production and use. Plant Soil 230:21–30
Deaker R, Roughley RJ, Kennedy IR (2004) Legume seed inoculation technology – a review. Soil Biol Biochem 36:1275–1288
Denarie J, Debelle F, Rosenberg C (1992) Signaling and host range variation in nodulation. Annu Rev Microbiol 46:497–531
De Bruijn FJ (1992) Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol 58:2180–2187
Lanier JE, Jordan DL, Spears JF, Wells R, Dewayne Johnson P (2005) Peanut response to inoculation and nitrogen fertilizer. Agronomy J 97:79–84
Löbler M, Hirsch AM (1993) A gene that encodes a proline-rich nodulin with limited homology to PsENOD12 is expressed in the invasion zone of Rhizobium meliloti-induced alfalfa nodules. Plant Physiol 103:21–30
López-García S, Vásquez TEE, Favelukes G, Lodeiro AR (2002) Rhizobial position as a main determinant in the problem of competition for nodulation in soybean. Environ Microbiol 4:216–224
Mathan N, Parani M, Parida A, Nair S (1996) Random amplified polymorphic DNA analysis of root-nodulating bacterial strains from Arachis hypogaea with physiological characteristics of both fast and slow growers. Lett Appl Microbiol 23:89–92
McDermott TR, Graham PH (1989) Bradyrhizobium japonicum inoculant mobility, nodule occupancy, and acetylene reduction in the soybean root system. Appl Environ Microbiol 55:2493–2498
Peoples MB, Herridge DF, Ladha JK (1995) Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production. Plant Soil 174:3–28
Schultze M, Kondorosi A (1998) Regulation of symbiotic root nodule development. Annu Rev Genet 32:33–57
Somasegaran P, Hoben HJ (1994) Handbook for Rhizobia. Methods in Legume-Rhizobium Technology. Springer-Verlag, New York
Taurian T, Ibañez F, Fabra A, Aguilar OM (2006) Genetic diversity of rhizobia nodulating Arachis hypogaea L. in central Argentinean soils. Plant Soil 282:41–52
Thies JE, Singleton PW, Bohlool BB (1991) Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced Rhizobia on field-grown legumes. Appl Environ Microbiol 57:19–28
Urtz BE, Elkan GH (1996) Genetic diversity among Bradyrhizobium isolates that efectively nodulate peanut (Arachis hypogaea). Can J Microbiol 188:65–75
van Rossum D, Schuurmans FP, Gillis M, Muyotcha A, Van Verseveld HW, Stouthamer AH, Boogerd FC (1995) Genetic and phenetic analyses of Bradyrhizobium strains nodulating peanut (Arachis hypogaea L.) roots. Appl Environ Microbiol 61:1599–1609
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 24:6823–6831
Vincent JM (1970) A manual for the practical study of root-nodule bacteria, IBP Handbook No. 15. Blackwell Scientific Publications, Oxford, UK
Wadisirisuk P, Danso SKA, Hardarson G, Bowen GD (1989) Influence of Bradyrhizobium japonicum location and movement on nodulation and nitrogen fixation in soybeans. Appl Environ Microbiol 55:1711–1716
Zhang X, Nick G, Kaijalainen S, Terefework Z, Paulin L, Tighe SW, Graham PH, Lindström K (1999) Phylogeny and diversity of Bradyrhizobium isolated from root nodules of peanut (Arachis hypogaea) in Sichuan, China. Syst Appl Microbiol 22:378–386
Acknowledgments
This work was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), and Síntesis Química SAIC. WG is a Career Member of CONICET. PB has a doctoral fellowship from CONICET. The authors thank Mr. G. Cerioni for providing field facilities in La Aguada, and Dr. S. Anderson for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bogino, P., Banchio, E., Bonfiglio, C. et al. Competitiveness of a Bradyrhizobium sp. Strain in Soils Containing Indigenous Rhizobia. Curr Microbiol 56, 66–72 (2008). https://doi.org/10.1007/s00284-007-9041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9041-4