Abstract
Biosorption has been shown to be an eco-friendly approach to remove heavy metal ions. In this study, the photosynthetic bacteria Rhodobacter capsulatus was screened and found to have strong ability to adsorb Au(III). The maximum specific uptake of living cells was over 92.43 mg HAuCl4/g dry weight of cell in the logarithmic phase. Biosorpion ability would be enhanced by an acidic environment. As the main cations, during biosorption the quantity of Mg2+ exchanged was more than Na+. Biosorbed Au(III) could be reduced by carotenoid and enzymes embedded and/or excreted by R. capsulatus, which might be the mechanism of photosynthtic bacteria metal tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is one of great environmental concerns because the heavy metals are nondegradable and persistent in water and soil [13]. There is no single chemical approach that can decrease, if not eliminate, all heavy metals, which makes the cost high. Also, the chemical ones are accompanied by new environmental problems. Therefore, a cost-effective and eco-friendly bioremediation strategy for heavy metal decontamination of waters and soil has been long sought. Biosorption is a process that utilizes living or nonliving biomass to sequester heavy metals, which has been shown to be an effective approach [1].
Photosynthetic bacteria (PSB), the typical aquatic organisms inhabiting anaerobic environments, have been applied in the field of environmental protection, such as the treatment of sewage and wastewaters [12, 14] and the bioremediation of the environment polluted with organic matters [16]. However, application of PSB in biosorption of heavy metal ion is rare.
Photosynthetic bacteria can produce reducing power to fix nitrogen and reduce the proton to H2. Most of the reports on the application of PSB in metal concern metal reduction [3, 9, 10, 15, 18]. However, few researches had been done on Au biosorption and reduction.
In this article, experiments were conducted to investigate the ability of Au(III) biosorption by Rhodobacter capsulatus under different conditions. In addition, bioreduction of Au(III) was exhibited extracellularly and the possible mechanism was elucidated.
Materials and Methods
Strains and Culture Conditions
The PSB R.capsulatus, a purple nonsulfur bacteria, were isolated and cultured by our laboratory and were used in subsequent experiments. The bacteria were grown in purple nonsulfur bacteria-enriched medium: 1.0 g NH4Cl, 0.5 g Na2HPO4, 0.2 g MgCl2, 2 g NaCl, 2 g yeast extract, 6 m L of 80% sodium lactate, dissolved in 1 L of distilled water; the pH was adjusted to 7.2 with NaOH before autoclaving. The bacteria were cultured anaerobically at 30°C under continuous illumination with incandescent lamps at a light intensity of about 2000 lux. Before the Au(III) biosorption experiment, the cell concentration was adjusted to 38 mg dry weight of cell per 10 mL cell suspension. Cells of two different growth phases (logarithmic and stationary phases) were collect to compare their Au(III) biosorption capabilities. All other conditions were kept the same.
Au(III) Biosorption Experiments
Effect of HAuCl4 Concentration on Biosorption
Every 10 mL of cell suspension was introduced into 40 mL of HAuCl4 solution. Experiments were carried out with a 50-mL cell–HAuCl4 solution at different initial concentrations ranging from 40 to 240 mg/L in 250-mL flasks. The flasks were incubated for 10 min in a rotary shaker at 150 rpm and 30°C. Then the mixture was centrifuged at 10,000 rpm for 10 min.
Effect of pH on Biosorption
A series of experiments was conducted at different pHs. The pH of 40-mL HAuCl4 solutions at 50 mg/L concentration was first adjusted to a specific value, from 1.0 to 9.0, and then a 10-mL cell suspension was introduced, followed by 10 min incubation.
Kinetic Experiments
In order to determine the kinetics of Au(III) removal from the solution, experiments were conducted at an initial concentration of 40 mg/L. A series of flasks was taken from the rotary shaker at predefined time intervals (2, 4, 10, 30, 60 min) to analyze the ion concentration. Each experiment was repeated three times.
Specific Metal Uptake
The specific Au(III) uptake q (mg/g dry weight of cell) was calculated using the \( q = (C_0 - C) \times v/m \), where C 0 is the initial Au(III) ion concentration (mg/L), C is the residual Au(III) ion concentration (mg/L), m is the dry weight of the cell (g), and v is the volume of the reaction mixture (L).
Analytical Techniques
Soluble Au, Mg, and Na ion concentrations were measured using an inductively coupled plasma spectrometer (ICP) (Thermo, USA). The cells with different incubating times were analyzed by transmission electron microscopy (JEOL, JEM-200EX) and scanning electron microscopy (Sirion; FEI, USA). The X-ray Energy Disperse Spectroscopy (EDAX, USA) analysis was used to identify the change of the main metal ions in the cell. The cell density was measured by an ultraviolet-visible spectrophotometer.
Results and Discussion
The Growth Curve of Photosynthetic Bacteria
In order to compare the ability of Au(III) biosorption of different growth phases, based on Fig. 1, cells in the logarithmic phase and stationary phase were collected.
Effect of Initial Au(III) Concentration on Biosorption
The initial HAuCl4 concentration had an impact on the biosorption ability of PSB, as shown in Fig. 2. The specific Au(III) uptake of R. capsulatus was facilitated by the higher concentration of HAuCl4 both in the logarithmic phase and stationary phase, but the earlier experiment on inhibitory concentrations showed that when the concentration of Au(III) was >80 ppm, the growth of R. capsulatus would be inhibited completely. Thus, the maximum specific uptake of the living cell was >92.43 mg HAuCl4/g dry weight of the cell (in the logarithmic phase). Although the specific uptake would continue to increase with a Au(III) concentration >80 ppm, and even reach 267 mg HAuCl4/g dry weight of cell, the structure of the cell had been destroyed by the high concentration of Au(III). All cell inclusion discharged could enhance the biosorption ability of cell, which was not discussed in this article.
Effect of Incubation Time on Biosorption
The strong ability of biosorbing Au(III) was found for PSB in both the logarithmic phase and stationary phase (shown in Fig. 3). Fast biosorption was observed for the first 30 min. At 2 min, biosorption of about 57% Au(III) had been removed from the solution. Also, 97.25% Au(III) had been biosorbed by PSB at 30 min. Longer contact times allowed a more thorough biosorption of Au(III). Nearly 100% Au(III) removal was observed for both phases when a 60-min incubation time was applied. However, slight differences of the biosorption abilities was found for the logarithmic phase and stationary phase.
As shown in Figs. 2 and 3, the ability of Au(III) biosorption of the logarithmic phase was stronger than the stationary phase.
Effect of pH on Biosorption
The cell wall consists of a variety of polysaccharides and proteins and, hence, offers a number of active sites capable of binding metal ions [7]. The potential metal-binding groups are carboxylate, amine, imidazole, phosphate, sulfhydryl, sulfate, and hydroxyl [2]. Nitrogen and oxygen of peptide bonds are also available for coordination bonding with metal ions. Such bond formation could be accompanied by the displacement of protons and is dependent in part on the extent of protonation, which is determined by the pH [4, 5, 11, 17].
The results indicated that the ability of Au(III) biosorption decreased sharply when the proton concentration in solution was lowered (shown in Fig. 4). The reason might be that AuCl −4 has a lower solubility and is thus adsorbable at a lower pH; on the other hand, the lower pH results in high concentrations of protons, which neutralizes the negative charge on R. capsulatus, which makes binding groups carry more positive charges at a low pH and strengthens the AuCl - 4 ion adsorption by means of electrostatic attractions and ion exchange.
Exchange of Mg and Na Ions During Biosorption
The reactive groups of the cell wall function as an ion exchanger, which desorb cations to biosorb heavy metal ions. The chloroaurate ion is not stable in solution [19], so the ion exchange might happen between the Au(III) dissociated from the chloroaurate ion biosorbed on the cell wall and previous ions of the cell wall [6]. Because the cations in the enriched medium were prevalently Mg and Na ions, analysis of these two ions were performed in particular to determine the varieties of them during the Au(III) biosorption. When the Au(III) concentration in solution decreased, the concentration of Mg and Na ions increased correspondingly. As shown in Fig. 5, after 2 min of contact, about 57% Au(III) had been removed and the concentration of Mg and Na ions increased sharply to about the 43% and 40% of the maximum desorbing concentration, respectively.
As shown in Fig. 6, with the increase of the initial HAuCl4 concentration, the quantity of Mg and Na ions desorbed also increased. Interestingly, at the initial HAuCl4 concentration of 80 mg/L, the quantity of exchanged Na ions reached its maximum, which corresponded to the inhibitory experiment, but the Mg ions desorbed continued to increase, especially in the stationary phase. These phenomena might result from the cell disruption caused by the high concentration of Au(III), which will be studied further in later research. In general, the quantity of Mg ions exchanged during biosorption was more than for Na ions.
Bioreduction of Au(III)
Rhodobacter capsulatus, a purple nonsulfur bacteria, can use light energy and/or chemical (organic and inorganic) energy to grow. Photophosphorylation and aerobic respiration can be used as alternative approaches to produce the reducing power. Carotenoid, which functions in the plasma membrane of PSB as a protecting substance to eliminate the oxygen-free radicals (OFRs) has a low potential.
When the AuCl −4 concentration was higher than 80 mg/L, the color of the bacteria changed from red to green as soon as AuCl −4 was added. A reasonable explanation might be that when the AuCl −4 ion concentration was higher than 80 mg/L, the quantity of AuCl −4 exceeded the maximum allowance of biosorption of the cell wall; therefore, the superfluous AuCl −4 ion crossed the cell wall quickly and was biosorbed by the plasma membrane. When these biosorbed metal ions harm the plasma membrane, carotenoid could prevent this from happening and protect the bacteria. When the trivalent Au was reduced, the color of the bacteria appeared as green, which was exhibited by bacteriochlorophyll and originally covered by the color of the carotenoid. The process of the carotenoid-reducing heavy metal ion on its plasma membrane could be deemed the self-protection of PSB.
To demonstrate its reducing ability, carotenoid was extracted from PSB by acetone and dissolved in petroleum ether. After a vigorous shake, the colors of the carotenoid and AuCl −4 solution disappeared and purple emerged, indicating the formation of gold nanoparticles.
After about 5 h incubation, the color of the solution turned from pale yellow to purple, and Au(III) was reduced extracellularly, as shown in Fig. 7A. Transmission electron microscopy showed the outline of R. capsulatus, on which there were many gold nanoparticles (shown in Fig. 8). Figure 7B shows the solution incubated after 24 h. After a long enough contact time, Au(III) could be reduced by NADP-dependent enzymes excreted extracellularly or protein of the electron transfer chain embedded in plasma. This could be the potential extracellular mechanism of metal tolerance [8].
X-ray EDS analyses showed the atoms’ quantity ratio of Na, Mg, and Au at different times. The ratio of the original is shown in Fig. 9A. The one after 2 min is shown in Fig. 9B. Figure 9C shows the ratio after 5 h. The comparison of the atom ratio of Au, Mg, and Na at different times (as shown in Fig. 9 and Table 1) resulted in the AuCl − 4 ion being biosorbed first by various reactive groups available on the cell surface; then some of the Au(III) could be reduced by the carotenoid or the enzyme embedded and/or excreted by bacteria.
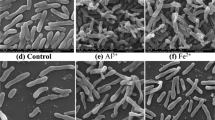
Effect of Au(III) on R. capsulatus’s Form
To study the effect of AuCl −4 on the form of R. capsulatus, scanning electron micrographs were taken with 40 mg/L of the initial HAuCl4 concentration. The original form of PSB was ovate (Fig. 10A). After 2 min contact with Au(III), the cell transformed distinctively (Fig. 10B). After 5 h, the distortion of cells had some amelioration (Fig. 10C). These phenomena supported the idea that Au(III) reduction could be the mechanism of photosynthetic bacteria metal tolerance. After the reduction of Au(III), the quantity of biosorbed Au(III) might decrease to a harmless level to bacteria.
Conclusion
The living R. capsulatus has strong biosorption ability for the AuCl −4 ion. For 38 mg dry weight of bacteria at 80 mg/L Au(III) concentration, in 10 min 88% of the AuCl −4 ions had been biosorbed. The maximum specific uptake of living cells was over 92.43 mg HAuCl4/g dry weight of the cell. The Au(III) biosorption of the logarithmic phase was better than the stationary phase. The lower pH value facilitated Au(III) biosorption of photosynthetic bacteria. Between the main exchanging cations, Mg2+ and Na+, the quantity of Mg2+ exchanged during biosorption was more than for Na+, based on which further research was conducted to focus on the influence of pretreatment and thus modification of the cell wall by cations on Au(III) biosorption of R. capsulatus. Biosorbed Au(III) would be reduced by a carotenoid and enzymes embedded and/or excreted by R. capsulatus, which could be the mechanism of photosynthetic bacteria metal tolerance. The ability of biosorption and bioreduction shown by photosynthetic bacteria could be useful in heavy metal removal and helpful in describing the phenomena caused by the valence change of metal ion.
References
Beveridge TJ, Murray RG (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141:876–887
Crist RH, Oberholser K, Shank N, Nguyen M (1981) Nature of bonding between metallic ions and algal cell walls. Environ Sci Technol 15:1212–1217
Ehrenreich A, Widdel F (1994) Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Envrion Microbiol 60:4517–4526
Friis N, Myers-Keith P (1986) Biosorption of uranium and lead by Streptomyces longwoodensis. Biotechnol Bioeng 27:21–28
Horikoshi T, Nakajima A, Sakaguchi T (1981) Studies on the accumulation of heavy metal elements in biological systems. Eur J Appl Microbiol Biotechnol 12:90–96
Bang H, Lee EJ, Lee EY, Suh J, Suh MP (2000) Transmetallation of nickel(II) ion in a nickel(II) azamacrocyclic complex with gold(III) ion. Inorg Chim Acta 308:150–154
Kuyucak N, Volesky B (1989) Biosorbents for recovery of metals from industrial solutions. Biotech Lett 33:823–831
Bellion M, Courbot M, Jacob C, Blaudez D, Chalot M (2006) Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol Lett 254:173–181
Moore MD, Kaplan S (1992) Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol 174:1505–1514
Moore MD, Kaplan S (1994) Members of the family Rhodospirillaceae reduce heavy-metal oxyanions to maintain redox poise during photosynthetic growth. Am Soc Microbiol News 60:17–23
Muraleedharan TR, Iyengar L, Venkobachar C (1991) Biosorption: an attractive alternative for metal removal and recovery. Curr Sci 61:379–385
Nagadomi H, Kitamura T, Watanabe M, Sasaki K (2000) Simultaneous removal of chemical oxygen demand (COD), phosphate, nitrate and hydrogen sulphide in the synthetic sewage wastewater using porous ceramic immobilized photosynthetic bacteria. Biotechnol Lett 22:1369–1374
Gupta R, Ahuja P, Khan S, Saxena RK, Mohapatra H (2000) Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr Sci 78(8):967–973
Sasaki K, Tanaka T, Nagai S (1998) Use of photosynthetic bacteria for production of SCP and chemicals from organic wastes. In: Martin AM (ed) Bioconversion of waste materials to industrial products, 2nd ed. Blackie Academic and Professionals, New York pp 247–291
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater Lett 61:3984–3987
Takeno K, Sasaki K, Nishio N (1999) Removal of phosphorus from oyster farm mud sediment using a photosynthetic bacterium, Rhodobacter shaeroides IL106. J Biosci Bioeng 88:410–415
Volesky B (1990) Biosorption of heavy metals. CRC Press, Boca Raton, FL
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362:834–836
Ran Y, Fu J, Rate AW, Gilkes RJ (2002) Adsorption of Au (I,III) complexes on Fe, Mn oxides and humic acid. Chem Geol 185:33–49
Acknowledgments
This work is part of the project (40571156) supported by National Natural Science Foundation of China. We are grateful to Professor Gangya Zhang from the Analysis and Testing Center of Institute of Soil Science, Chinese Academy of Sciences for kindly helping with measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Y., Yu, Y., Wang, Y. et al. Biosorption and Bioreduction of Trivalent Aurum by Photosynthetic Bacteria Rhodobacter capsulatus . Curr Microbiol 55, 402–408 (2007). https://doi.org/10.1007/s00284-007-9007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9007-6