Abstract
This study reports the regulation of multiple xylanases produced by Myceliophthora sp. IMI 387099. Fructose was found to positively regulate the expression of multiple xylanase when used as sole carbon source. The xylanases (EX1 and EX2) of acidic pI were expressed in the presence of simple sugars (glucose, arabinose, and xylose), whereas xylanase of both acidic as well as basic pI (EX1, EX2, EX3, and EX5) were expressed in the presence of fructose, xylan, and combination of xylan and alcohol. The combination of fructose and xylan also led to expression of an additional xylanase (EX4). The positional isomer (iso-X4) was found to be the key transglycosylation product when cultures were grown in the presence of fructose and xylan. In the presence of alcohols, the higher expression of xylanase was ascribed to the synergistic effect of alkyl glycoside and other transglycosylation products present in the culture extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
β-1, 4-Endoxylanase (EC 3.2.1.8) is one of the key components of hemicellulases involved in the hydrolysis of xylan backbone present in different heteropolysaccharides. These xylanases are produced by a number of fungi and bacteria during growth on complex lignocellulosic substrates [1]. A variety of compounds have been identified as inducers of xylanase production, i.e., xylan and xylo-oligosaccharides, mainly xylotrioses, xylobiose, and xylose. In addition, lactose, L-sorbose, and sophorose have also been reported to induce xylanase produced in different organisms [2, 3]. Because polymeric forms of cell wall components, i.e., xylan and cellulose, are unable to enter the fungal cell, it has been suggested that low molecular-weight hydrolysis products of xylan and cellulose penetrate the cell and induce the production of hydrolytic enzymes [4]. The role of transglycosylating enzymes in the synthesis of positional isomers (aldopentauronic acid, isomeric xylotetraose, and isomeric xylotriose) as inducer molecules has also been reported in Schizophyllum commune [5]. Once produced, these inducer molecules are translocated rapidly into the cell by permease–transferase systems. The affinity of uptake systems toward inducers plays an important role in the induction mechanism. Many of the microbes, including fungi and bacteria, produce multiple xylanases that are functionally diverse and have been classified into different hydrolase families number 5, 7, 8, 10, 11, and 43. Molecular evidence suggests that the regulation of expression of xylanases from different families differs markedly from family to family [6]. Therefore, gaining insight into regulation of expression of these xylanases is important from the basic as well as applied aspects. This study reports differences in regulatory mechanisms for the expression of multiple xylanases in a thermophilic fungal strain of Myceliophthora sp.
Materials and Methods
Culture and growth medium
The thermophilic fungus isolated from composting soils and identified as Myceliophthora sp. IMI 387799, as described previously [7], was taken up for studying the regulation of expression of multiple xylanase. The culture was grown and maintained on yeast starch agar (YpSs, pH 7.0) [8].
For preparation of inoculum, culture was grown in 250-ml Erlenmeyer flask containing 50 ml glucose medium with the following composition (% w/v): glucose 1.0; yeast extract 1.0; K2HPO4 0.04; and MgSO4 · 7H2O 0.05. The pH of the medium was adjusted to 7.0, and flasks were incubated under shaking conditions (120 rpm) at 45°C for 60 hours. The mycelium was collected by filtration under sterile conditions, washed, and transferred to fresh induction medium.
Regulation of xylanase
For studying the regulation, the production medium (100 ml)―which contained (NH4)2SO4 0.3%; KH2PO4 0.4%; ammonium acetate 0.6%; and 1% (w/v) monosaccharides (glucose, fructose, arabinose, xylose); polysaccharides (oat spelt xylan); or 1% (v/v) alcohols (ethanol, propanol, and methanol)―was inoculated with the washed mycelium (2 g wet weight) from 60-hour old culture grown on glucose medium. For studying possible repressive or synergistic effects, different monosaccharides (1% w/v) or alcohols (1% v/v) were added to xylan (0.5% w/v oat spelt xylan) containing production medium. The initial pH of the medium was adjusted to 7.0. The flasks were incubated at 45°C under shaking conditions (120 rpm) for up to 120 hours. The samples were withdrawn at 12-hour interval up to 120 hours and centrifuged (11,000 × g for 10 minutes), and supernatants were assayed for xylanase activity.
Enzyme assay
Xylanase activity was determined using birch wood xylan 1% (w/v) prepared in sodium citrate buffer (50 mM, pH 6.0) [9]. An equal amount of suitably diluted enzyme and substrate was incubated at 50°C for 5 minutes. After incubation, the reaction was stopped by addition of dinitro salicylic acid followed by boiling [10]. The developed color was monitored at 540 nm using Novospec II spectrophotometer (Pharmacia). The amounts of released sugars were quantified using the xylose standard. Protein in the enzyme extracts was determined by the protein dye-binding method as described by Bradford [11].
Isoelectricfocusing and activity staining
Isoelectricfocusing (IEF) was performed as described previously [12] using 5% acrylamide gel containing 2.4% broad pH range (3.5 to 10.0) ampholine carrier ampholyte (Amersham Biosciences). The cathode buffer contained ethanolamine 0.4% (v/v), and sulphuric acid, 0.2% (v/v), was used as anode buffer. Xylanase activity in IEF gels was detected by activity staining with agarose replica gels containing covalently dyed RBB-xylan as described by Biely et al [13].
Detection of transglycosylation products by thin-layer chromatography
The culture extract samples collected at different time intervals were concentrated by lyophilization and analyzed for the presence of hydrolysis and transglycosylation products by thin-layer chromatography (TLC) on microcrystalline cellulose (Merck) plates. The plates were developed twice in the solvent system ethyl acetate–acetic acid–water (3:2:1). Reducing sugars were detected using the aniline-hydrogen phthalate reagent as described previously [14, 15].
Results
The inductive effect of xylan on xylanase production by Myceliophthora sp. was studied. Different monosaccharides, e.g., glucose, fructose, xylose, and arabinose as well as a combination of xylan and monosaccharides, were used to study their regulatory effects on the expression of multiple xylanases (Fig. 1a). The production profile showed that an initial detectable induction of xylanase after 24 hours was observed in the presence of xylan as well as xylan plus arabinose. Whereas, a rapid increase in xylanase expression was observed in medium containing fructose or fructose and xylan, at 48 hours when the observed xylanase levels were comparatively higher than those in xylan plus arabinose and appreciably higher than in culture grown on arabinose or glucose as sole carbon source in the medium. The levels of xylanase increased steadily up to 96 hours in the presence of xylan and xylan plus fructose, but a sharp decrease in xylanase was observed in culture grown in fructose. Furthermore, cultures grown in the presence of xylan, in combination with arabinose or glucose, respectively, showed moderate to relatively higher repression of xylanase, although no repression was observed when fructose was added to xylan, which in fact registered higher levels of xylanase (3.92 U/ml) compared with those (3.76 U/ml) obtained on xylan alone. Similarly, the addition of glycerol showed no repressive effects (Fig. 1c).
The results showed that the addition of alcohols to xylan-culture media resulted in early and higher induction of xylanase within 24 hours as compared to medium containing xylan (Fig. 1b). The presence of methanol, ethanol, and propanol resulted in 1.94, 1.16, and 1.23 U/ml xylanase activity, respectively, within 24 hours of incubation, and was 3.3, 2.0, and 2.1-fold higher than 0.574 U/ml xylanase produced by xylan alone.
The zymograms developed against proteins resolved by IEF showed differential expression of multiple xylanase isoforms in the presence of the different carbon sources used (Fig. 2). The presence of arabinose, xylose, cellobiose, and glucose in medium resulted in the expression of only two xylanases with acidic pI (EX1 and EX2). However, fructose or xylan, when used as sole carbon sources, induced four iso-xylanases, namely, EX1, EX2, EX3, and EX5. However, fructose in combination with xylan, which was previously observed to induce maximal xylanase production after 96 hours, expressed five different xylanase isoforms, i.e., EX3, EX4, and EX5 with basic pI, in addition to EX1 and EX2. The zymogram (Fig. 2b) showed that all the four iso-xylanases expressed in the presence of xylan were also present in the extracts from cultures grown in presence of alcohols plus xylan.
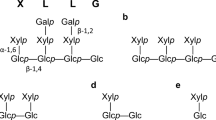
TLC profiling (Fig. 3) showed the presence of different transglycosylation products in culture extracts of arabinose, cellobiose, glucose, xylose, or fructose taken at different time intervals. In the presence of arabinose, cellobiose, glucose or xylose, a transglycosylation product in the form of an oligosaccharide greater than X5 was observed after 24 hours, and this product persisted in the medium without being further metabolized. In addition, smaller oligomeric transglycosylation products of arabinose, glucose, and xylose were also observed. Interestingly, the observed transglycosylation product in the presence of fructose was different from that observed for other monosaccharides in that it possibly was a positional isomer with Rf corresponding to iso-X4. A similar transglycosylation–hydrolysis product was also observed in the presence of xylan. In the presence of fructose, maximal xylanase activity was observed after 48 hours of incubation followed by a decrease in the activity. Thereafter, the decrease in the activity corroborated well with the decreased levels of this positional isomer (iso-X4) from the culture extracts. However, the accumulation of this product (iso-X4) continued for up to 72 hours in the presence of xylan as well as a combination of xylan plus fructose, and this correlated well with increased xylanase production. In the presence of alcohols, a combination of transglycosylation–hydrolysis products, ranging from higher to lower oligomers and alkyl glycosides in the culture extracts, possibly induced comparatively higher expression of xylanase (data not shown).
TLC profiling showed the presence of different hydrolysis and transglycosylation products in culture extracts taken at different time intervals. S, standards (X1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetrose; X5, xylopentose); A, arabinose; C, cellobiose; G, glucose; x, xylose; F, fructose; X, xylan; X + F, xylan + fructose.
Discussion
Multiplicity of xylanases is a common phenomenon in micro-organisms [16], including thermophilic fungi, i.e., Melanocarpus albomyces, Myceliophthora, sp. and Humicola insolens [17, 12], where each micro-organism is capable of expressing diverse xylanases belonging to different families. However, the phenomenon behind the differential expression of these multiple isoxylanases is not well understood. In this article, we report the regulation of expression of multiple xylanases from Myceliophthora sp., which has been previously shown to be functionally diverse [12]. The results of this study suggested that Myceliophthora sp., when grown in the presence of glucose, arabinose, xylose, and cellobiose, expressed xylanases (EX1 and EX2) of acidic pI that have been previously characterized to be xylanases belonging to family number 10, with broad substrate specificity [12, 18]. The expression of xylanase with broad substrate specificity may be a possible mechanism adopted by this fungus for supporting initial growth on a wide variety of xylan types, from diverse plant sources to composting material available in nature. In contrast, the inducible xylanase isoforms, EX3, EX4, and EX5, with basic pI, that have been classified as members of family number 11 [12], were expressed in the presence of fructose, xylan, and the combination of fructose plus xylan. The differential expression of xylanases of families number 10 and 11 may also be attributed to basic differences in the molecular organization of promoter regions [6].
The results of this present study suggested a definite role of transglycosylation products in the expression of multiple isoxylanases produced by Myceliophthora sp. The presence of glycosyl transferase activity in the purified xylanases from Myceliophthora sp. has been previously demonstrated [7], where the products of hydrolysis acted as a donor or acceptor during the reaction. Interestingly, TLC results suggested that in response to glucose, cellobiose, arabinose, and xylose, a transglycosylation product of approximately Rf X5 is formed, which is possibly not recognized by members of family number 10 xylanases [18, 19]. Therefore, these products persist in the culture filtrates. In contrast, inducible xylanases belonging to family number 11 recognize the formed product as substrate and are cleaved to shorter–chain-length oligomers, resulting in formation of some of the positional isomers, i.e., iso-X4, which were found to be the key transglycosylation products identified in the presence of fructose, xylan, and their combinations (conditions that supported maximum xylanase activity). However, differences in the structure of putative isomeric products formed (F-X3) in the presence of xylan plus fructose, xylan (iso-X4), or fructose (F4), and their ability for differential expression of xylanases, cannot be ruled out because transglycosylation product (F-X3) resulted in induction of an additional xylanase isoform, i.e., EX4. Fructose representing ketose sugar has also been observed to positively regulate cellulase production in another thermophilic fungus, Melanocarpus sp [20].
Furthermore, in the presence of alcohols, appreciably higher induction was observed, although the number of xylanase isoforms in the presence of alcohols was same as that observed in xylan. The inductive effect of alcohols may be caused by formation of alkyl glycosides (in addition to other transglycosylation products), which has been shown to induce polysaccharidase activity [21]. Moreover, the role of methyl β-D-glycoside has also been observed in the expression of multiple xylanases in Neocallimastix frontalis [22].
This study clearly showed specific roles of fructose and transglycolsylation products in the regulation of multiple xylanases in Myceliophthora sp. The metabolic profiling carried out in this study substantiated previous observations on the expression of xylanases of family numbers 10 and 11 at the molecular level [6].
Literature Cited
Purkarthofer H, Steiner W (1995) Induction of endo-β-xylanase in the fungus Thermomyces lanuginosus. Enzyme Microbiol Technol 17:114–118
Zeilinger S, Mach RL, Schindler M, Herzog P, Kubicek CP (1996) Different inducibility of expression of the two xylanase genes xyn1 and xyn2 in Trichoderma reesei. J Biol Chem 271:25624–25629
Xu J, Takakuwa N, Nogawa M, Okada H, Morikawa Y (1998) A third xylanase from Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol 49:718–724
Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S (1996) Production of fungal xylanases. Bioresour Technol 58:137–161
Kolenova K, Vrsanska M, Biely P (2005) Purification and characterization of two minor endo-β-1, 4-xylanases of Schizophyllum commune. Enzyme Microbiol Technol 36:903–910
Chavez R, Schachter K, Navarro C, Peirano A, Aguirre C, Bull P, et al. (2002) Differences in expression of two endoxylanase genes (xyn A and xyn B) from Penicillium purpurogenum. Gene 293:161–168
Chadha BS, Badhan AK, Mellon F, Bhat MK (2004) Two endoxylanases active and stable at alkaline pH from the newly isolated thermophilic fungus, Myceliophthora sp. IMI 387099. J Biotechnol 104:227–237
Cooney DC, Emerson R (1964) Thermophilic fungi: An account of their biology, activities and characterization. San Francisco, CA: Freeman, pp 1–188
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing methods for assay of xylanase activity. J Biotechnol 23:257–270
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of decreasing sugars. Anal Chem 31:426–428
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Badhan AK, Chadha BS, Sonia KG, Saini HS, Bhat MK (2004) Functionally diverse multiple xylanases of thermophilic fungus Myceliophthora sp. IMI 387099. Enzyme Microbiol Technol 35:460–466
Biely PD, Mislovicova, toman B (1988) Remazol brilliant blue―A soluble chromogenic substrate for xylanases. In: Wood T, Kellogg ST (eds) Methods in enzymology, vol. 160. London, UK: Academic, pp 536–541
Bennett NA, Ryan J, Biely P, Vrsanska M, Kremnicky L, Macris BJ, et al. (1998) Biochemical and catalytic properties of an endoxylanases purified from the culture filtrate of Thermomyces lanuginosus ATCC 46882. Carbohydr Res 306:445–455
Martin FC (1986) Monosaccharides. In: Chaplin MF, Kennedy JF (eds) Carbohydrate analysis, a practical approach. Washington, DC: IRL Press, pp 1–36
Wong KKY, Tan LUL, Saddler JN (1988) Multiplicity of β-1, 4-xylanase in microorganisms: function and applications. Microbiol Rev 52:305–317
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: Their physiology and enzymes. Microbiol Mol Biol Rev 63:461–488
Biely P, Vrsanska M, Tenkanen M, Kluepfel D (1999) Endo-β-1, 4-xylanase families: Differences in catalytic properties. J Biotechnol 57:151–166
Kolenova K, Vrsanska M, Biely P (2006) Mode of action of endo-1-4-β-xylanases of families 10 and 11 on acidic xylooligosacchrides. J Biotechnol 121:338–345
Jatinder K, Chadha BS, Saini HS (2006) Regulation of cellulase production in two thermophilic fungi Melanocarpus sp. MTCC 3922 and Scytalidium thermophilum MTCC 4520. Enzyme Microbiol Technol 38:931–936
Parry NJ, Beever DE, Owen E, Vandenberghe VBJ, Bhat MK (2001) Biochemical characterization and mechanism of action of a thermostable β-glucosidase purified from Thermoascus aurantiacus. Biochem J 353:117–127
de Gomez Segura B, Durand R, Fevre M (1998) Multiplicity and expression of xylanase in the rumen fungus Neocallimastix frontalis. FEMS Microbiol Lett 164:47–53
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badhan, A.K., Chadha, B.S., Kaur, J. et al. Role of Transglycosylation Products in the Expression of Multiple Xylanases in Myceliophthora sp. IMI 387099. Curr Microbiol 54, 405–409 (2007). https://doi.org/10.1007/s00284-006-0204-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0204-5