Abstract
A novel thermoacidophilic iron-reducing Archaeon, strain NA−1, was isolated from a hot fumarole in Manza, Japan. Strain NA-1 could grow autotrophically using H2 or S0 as an electron donor and Fe3+ as an electron acceptor, and also could grow heterotrophically using some organic compounds. Fe3+ and O2 served as electron acceptors for growth. However, S0, NO3 −, NO2 −, SO4 2−, Mn4+, fumarate, and Fe2O3 did not serve as electron acceptors. The ranges of growth temperature and pH were 60–90°C (optimum: 80°C) and pH 1.0–5.0 (optimum: pH 1.2–1.5), respectively. Cells were nearly regular cocci with an envelope comprised of the cytoplasmic membrane and a single outer S-layer. The crenarchaeal-specific quinone (cardariellaquinone) was detected, and the genomic DNA G + C content was 29.9 mol%. From 16S rDNA analysis, it was determined that strain NA-1 is closely related to Acidianus ambivalens (93.1%) and Acidianus infernus (93.0%). However, differences revealed by phylogenetic and phenotypic analyses clearly show that strain NA-1 represents a new species, Acidianus manzaensis, sp. nov., making it the first identified thermoacidophilic iron-reducing microorganism (strain NA-1T = NBRC 100595 = ATCC BAA 1057).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Dissimilatory microbial iron reduction is thought to play an important role in mineral formation in a variety of environments like subsurface soils, lake sediments, ground water, hot springs, and deep sea hydrothermal vents [10]. Until now, a number of iron-reducing microorganisms have been isolated, and found to be widely distributed in phylogenetic positions among Bacteria and Archaea [14]. On the basis of optimum pH and temperature for growth, they can be classified into the following three groups; (i) iron-reducing bacteria mainly belonging to the Proteobacteria such as Geobacter sulfurreducens [2] and Shewanella putrefaciens [16], which grow by the reduction of insoluble iron compounds under neutral pH conditions at around 30°C; (ii) thermophilic iron-reducing microorganisms such as Geothermobacterium ferrireducens [8], Geoglobus ahangari [11] and Pyrobaculum islandicum [9], which grow by iron reduction under neutral pH conditions in a thermal environment over 70°C; and (iii) acidophilic iron-reducing bacteria such as Acidithiobacillus ferreoxidans, growing by anaerobic iron respiration under acidic conditions at around 30°C through the reduction of soluble iron (Fe3+) by the oxidation of H2 or S0 [17]. However, there have been no reports on iron-reducing microorganisms growing at low pH and high temperature. Here we describe the first isolation and characterization of an acidothermophilic iron-reducing Archaeon, Acidianus manzaensis strain NA-1, which can chemolithoautotrophically grow through the reduction of Fe3+ by the oxidation of H2.
Materials and Methods
Isolation and culture conditions
The soil was sampled from a fumarole (85–94°C, pH 1.0–2.0) in Manza, Gunma, Japan. The iron-reducing (IR) basal medium contained (L−1): 0.132 g:(NH4)2SO4, 0.052 g:KCl, 0.041 g:K2HPO4, 0.490 g:MgSO4·7H2O, 0.009 g: CaCl2·2H2O, 0.001 g:ZnSO4·7H2O, 0.002 g:CuSO4·5H2O, 0.001 g: MnSO4·5H2O, 0.0005 g:NaMoO4·2H2O, 0.0005 g:CoCl2·6H2O, 0.001 g:NiCl2·6H2O, and 0.005 g:FeSO4·7H2O. In addition, 14.7 g of Fe2(SO4)3·nH2O was added as an electron acceptor. The pH of the medium was adjusted to 1.5 with 10 N H2SO4. Four hundred milliliters of the medium was added to 1-L anaerobic bottles, which were then sealed with a silicon cap. The head space gas was packed with H2/CO2 (4:1 (v/v)) to 150 kPa. After suspending a small amount of the soil in IR medium, it was injected into an anaerobic culture bottle prepared as described above and cultivated at 70°C while shaking (80 rpm). The concentrations of Fe2+ were measured using o-phenanthroline as described previously by Ohmura [17], and cells were counted under a light microscope.
Electron donors and acceptors
The growth potential of stain NA-1 was tested with a variety of combinations of potential electron donors and acceptors. All experiments were performed using 30 ml of IR basal medium in 120-ml anaerobic bottles. The 22 electron donors including (L−1) 1.6 g:S0, 3.0 g:potassium tetrathionate, 5.0 g:FeSO4·7H2O, 1.0 g: yeast extract, 5.5 g:sodium pyruvate, 8.2 g:sodium acetate, 8.9 g: sodium L-malate, 16.2 g:disodium succinate, 1.0 g:peptone, 6.8 g: sodium formate. 8.0 g:sodium fumarate, 1.0 g:glycerol, 1.0 g:sodium lactate, 29.4 g:sodium citrate, 3.0 g:glycine, 1.0 g:tryptone, 1.0 g:casamino acids, 1.0 g:beef extract, 1.0 g:sucrose, 1.0 g:mannose, 1.0 g:lactose monohydrate, and 1.0 g:glucose were tested. The concentration of each donor was adjusted by following the report by Kashefi et al. [8]. In the same manner, the nine electron acceptors for anaerobic respiration were individually tested at 14.7 g/L:Fe2(SO4)3·nH2O, 12.4 g/L:Fe(III)-citrate, 8.0g/L:Fe2O3 (crystalline iron oxide), 5.3 g/L:Fe(OH) 3, 14.7 g/L:SO4 2− as Na2SO4, 1.6 g/L:S0, 50 mM:NO3 − as NaNO3, 50 mM:NO2 − as NaNO2, 50 mM:Mn4+ as MnO2 and 50 mM:fumarate.
Optimal temperature, pH, salt, and concentration of O2
The potential for growth was tested using H2 as an electron donor, Fe3+ as an electron acceptor, and CO2 as a carbon source at various temperatures and various pH values while shaking (80 rpm). The temperature was varied within a range of 50–95°C in a hot-air incubator. The pH was varied within a range of pH 0.8–6.0, and growth occurring in the range of pH 2.0–6.0 was also investigated under aerobic conditions in which 10% O2 was added and Fe3+ was omitted. The potential for growth at various concentrations of Fe3+ or O2 was also investigated. The concentration of Fe3+ was varied from 0 to 150 mM, while the concentration of O2 was set by varying its proportion in the H2/CO2/O2 (4:1:X (v/v/v), where X defines the proportion of O2 in the total gas; 150 kPa,).
Electron microscopy
Cells in the late-exponential phase fixated with 4.0% glutaraldehyde for 12 h and dehydrated by ethanol, were observed through scanning electron microscopy (S-4500, HITACHI, Japan). Thin sections of the cells were prepared by rapid freezing and substitution fixation as described by Dempsey [4], and examined under a transmission electron microscope (H-7500; HITACHI, Japan).
Quinone analysis
Quinones were extracted using a mixture of chloroform and methanol (2:1 (v/v)) and identified by HPLC (LC-10; Shimadzu, Japan) using a reverse-phase column (ZORBAX-ODS; Shimadzu, Japan) as described by Collins [3].
Determination of the DNA G + C content
The G + C content was determined by reverse-HPLC (LC-10; Shimadzu, Japan) using a Develosil RPAQUEOUS column (4.6 mm × 250 mm; Nomura Chemical, Japan) as described by Katayama-Fujimura et al. [12].
16S rDNA sequence analysis
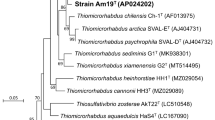
The 16S rDNA from strain NA-1 was determined using the archaeal-specific primers ARC20F (5′-TTCCGGTTGATCCYGCCRG-3′) and Uni1500R (5′-GGTTACCT TGTTACGACTT-3′) [5]. The sequence obtained from strain NA-1 was then subjected to a BLAST homology search (DDBJ gene bank, Japan), and the 16S rDNA sequences of close relatives and some typical archaea (listed in Fig. 5). These were aligned for phylogenetic analysis using Clustal X version 1.83 [19]. A phylogenic tree was then constructed with MEGA version 2.1 [13].
Results
Isolation of strain NA-1
Microorganisms in different soil samples were enriched by culture in an IR medium containing Fe3+ under an anaerobic atmosphere of H2/CO2 (4:1(v/v), 150 kPa) at 70°C and pH 1.5. The cultures showing Fe3+ reduction after 7–8 days were subcultured under the same conditions more than ten times. Samples of the microorganisms were then cultured on IR medium plates containing 3.0% gellan gum and Fe3+ under H2/CO2 (4:1(v/v), 150 kPa) at 70°C, and after 5–7 days colonies appeared. After repeating the colony isolation, strain NA-1 was isolated in a pure state.
Growth on iron respiration
Cell numbers increased from 1.0 × 106 to 1.0 × 108 cells/ml over a period of 150 h (Fig. 1A), and the growth correlated with the reduction of Fe3+ (Fig. 1B). No growth was observed in the absence of Fe3+, H2 or CO2, and there was no reduction of Fe3+ without cells. Thus, strain NA-1 is clearly capable of chemolithoautotrophic growth on the oxidation of H2 by the reduction of Fe3+. Growth on anaerobic iron respiration was confirmed by its dependence on the concentration of the electron acceptor in the medium. When strain NA-1 was inoculated into a medium containing 10-60 mM Fe3+, the cell density after 1 week of incubation was approximately proportional to the initial Fe3+ concentration (Fig. 2). This confirms that strain NA-1 is able to grow autotrophically using H2 as an electron donor and Fe3+ as an electron acceptor.
Chemolithoautotrophic growth of strain NA-1 on the anaerobic reduction of Fe3+ by the oxidation of H2. Strain NA-1 was cultivated at 70°C and pH 1.5. A: Cell numbers in the presence of H2 + CO2 + Fe3+ (•), H2 + CO2(▲), H2 + Fe3+ (▪), and CO2 + Fe3+ (♦). B: The concentrations of Fe3+ (•) and Fe2+(▪) in the presence of H2, CO2, and Fe3 +.
Utilization of electron donors and acceptors
S0, yeast extract, pyruvate, peptone, tryptone, casamino acid, sucrose, mannose, lactose, and glucose were all capable of supporting anaerobic growth as electron donors with the reduction of Fe3+. Fe3+ and O2 served as electron acceptors for growth. However, S0, NO3 −, NO2 −, SO4 2−, Mn4+, and fumarate did not serve as electron acceptors. Furthermore, strain NA-1 could utilize Fe2(SO4)3, Fe3+-citrate, or Fe(OH)3, but not Fe2O3 (crystalline iron oxide) in the anaerobic iron reduction.
Electron microscopy
The cells appeared as regular cocci (Fig. 3A), about 0.6–1.0 μm in diameter under transmission electron microscopy of thin sections (Fig. 3B). Figure 3C indicated that the cell envelope was composed of a cytoplasmic membrane, a periplasmic space, and a single outer S-layer, which is typical of Crenarchaea. In addition, the cells contained caldariellaquinone, which is a quinone found specifically in Crenarchaea.
Cells of strain NA-1 grown chemolithoautotrophically on anaerobic iron respiration. (A)Scanning electron micrograph; (B) Representative electron micrograph of a thin section of cells. (C) Higher magnification of membrane region of the cell boxed in B. CM, cytoplasmic membrane; PS, periplasmic space; SL, S-layer. Bars = 0.5 μm.
Optimum temperature, pH, salt concentration, and O2 partial pressure
Growth was observed at temperatures between 60 and 90°C, with the optimum at 80°C (Fig. 4A). No growth and no reduction of Fe3+ were observed below 55°C or at 94–95°C. The pH range for anaerobic iron-based growth was 1.0–2.0, with the optimum at 1.2–1.5 (Fig. 4B). In addition, the pH range for aerobic growth was 1.0–5.0, with the optimum at 1.2–2.0. No growth was detected at pH 5.5 or above. The growth was observed within a range of 1.0–20.0% O2 partial pressure, with the optimum at 5.0% (Fig. 4C).
Optical temperature, pH, and O2 on growth of strain NA-1. (A) Optical growth temperature of strain NA-1 under an anaerobic condition of H2, CO2, and Fe3+. (B) Influence of pH on the growth of strain NA-1. Fe3+ (•) or O2 (▼) was supplied as an electron acceptor (•). (C) Effect of O2 on aerobic growth on H2.
DNA base composition and evolutional analysis
The genomic DNA G + C content of strain NA-1 was 29.9 mol%. Phylogenetic analysis based on 16S rDNA indicated that the closest relatives of strain NA-1 are Acidianus ambivalens (93.1% similarity), A. infernus (93.0% similarity), A. brierleyi (89.9% similarity), and A. tengchongensis (89.9% similarity). which all belong to the archaeal genus Acidianus within the order Sulfolobales. The phylogenetic tree, determined by comparing 1358 bases from strain NA-1 with those of other archaeal species, showed that strain NA-1, Acidianus infernus and the other Acidianus strains clustered, suggesting strain NA-1 is a new species in the genus Acidianus (Fig. 5).
Discussion
Proposal of strain NA-1 as a new species of the genus Acidianus
Some phenotypic characteristics of strain NA-1 were the same as those of the four species in the genus Acidianus in its acidophilic and thermophilic growth: aerobic oxidation of H2 or S0 as an electron donor; the morphologic shape of cocci; the morphology of the cell envelope consisting of an S-layer attached to the cytoplasmic membrane; the presence of caldariellaquinone, which is a quinone found specifically in Crenarchaea; and the G + C content of the DNA as 29.9 mol% (Table 1) [1, 6, 7, 18]. These common characteristics between strain NA-1 and the other Acidianus species suggested that strain NA-1 belongs to genus Acidianus in the cluster II of the order Sulfolobales. Furthermore, low similarities of strain NA-1 and Acidianus species based on the 16S rDNA gene sequences suggested that strain NA-1 differs from the closest relatives, A. ambivalens, and A. infernus. The phylogenetic tree shows that strain NA-1 forms a branch together with Acidianus and Metallosphaera species. However, these two genera are physiologically different in terms of their aerobic or anaerobic growth. Acidianus species can grow aerobically or anaerobically, while Metallosphaera species can grow obligately aerobically, like Sulfolobus species. Furthermore, the mol% G + C contents of Acidianus or Metallosphaera species as around 31 or 45% suggests that these two genera were completely separated. Based on these classifications, faculative anaerobic growth and the 29.9 mol% of G + C contents in strain NA-1 clearly show that this strain belongs to genus Acidianus. Therefore, strain NA-1 should be classified to genus Acidianus, not to Metallosphaera, though strain NA-1 forms a cluster branching to these two genera. Additionally, the genus Acidianus is split into two monophyletic groups based on 16S rDNA analysis (Fig. 5). This split has no correlation with physiological classification by physiological characters of electron donor utility and autotrophy, which suggests the genus could be split into two groups as one group composed of A. infernus, A. ambivalens, and A. tengchongensis, the other of strain NA-1 and A. brierleyi. Therefore, Acidianus species should be reclassified in the near future.
Moreover, differences in metabolism were seen as strain NA-1 and A. brierleyi were both able to use the tested organic compounds as electron donors or carbon sources, while the other three Acidianus species were not able to do so. It is also particularly noteworthy that A. infernus, a close relative to strain NA-1, is an obligate autotroph; in contrast, strain NA-1 represented facultatively autotrophic growth under aerobic or anaerobic respiration. The most notable differences between strain NA-1 and the other Acidianus species are that strain NA-1 can grow autotrophically on the reduction of Fe3+ by the oxidation of H2, while the other Acidianus can not grow by Fe3+ reduction utilizing H2 or S0, which was experimentally confirmed by anaerobic growth and Fe3+ reduction in A. infernus, A. ambivalens, and A. brierleyi, or by the report of A. tengchongensis [7]. Furthermore, all of the four Acidianus species are capable of anaerobic utilization of S0 as an electron acceptor with the oxidation of H2, but strain NA-1 is not. These differences suggest that strain NA-1 should be classified as a novel species. We, therefore, propose that strain NA-1 is a new species of the genus Acidianus, which we have named Acidianus manzaensis (= NBRC 100595 = ATCC BAA 1057).
Anaerobic Metabolisms in Strain NA-1
One of the most distinguishing characteristics of strain NA-1 is its chemolithoautotrophic growth on the reduction of Fe3+ by the oxidation of H2. This metabolism in strain NA-1 is consistent with Lovley’s proposal that the ability for iron reduction coupled with H2 oxidation is much more universal among thermophilic microorganisms [14], and similar to the ancient metabolism in pre-biotic Earth proposed by Martin and Russell [15]. However, the utilization of soluble iron, not insoluble iron oxides in strain NA-1, is different from the hypothesized reaction. At this point, comparison between the biological aspects of strain NA-1 and those of other thermophilic iron-reducing microorganisms should be performed. Another distinguishing characteristic of strain NA-1 is the oxidization of S0 as an electron donor with the reduction of Fe3+, which is also the first case among Archaea. There is only one report on sulfur-oxidizing and iron-reducing microorganisms, acidophilic iron-reducing bacterium, Acidothiobacillus ferrooxidans. This bacterium grows autotrophically on the reduction of soluble iron by the oxidation of S0 or H2 [17]. In summary, we successfully isolated and characterized a novel thermoacidophilic iron-reducing archaeron, strain NA-1 growing autotrophically by the oxidation of H2 with the reduction of Fe3+. This is the first case in which iron reduction plays an important role even in extreme acidic and thermal conditions. More details about an iron-reducing mechanism in strain NA-1 are necessary to better understand dissimilatory iron reduction.
Description of Acidianus manzaensis sp. nov
Acidianus manzaensis (manzaen’sis. M.L. masc. adj. manzaensis, pertaining to Manza, Japan) is isolated from a hot fumarole in Manza, Japan. It is a facultative autotroph capable of both anaerobic and aerobic growth. H2, S0, yeast extract, pyruvate, peptone, tryptone, cassamino acids, sucrose, mannose, lactose, and glucose can serve as electron donors using Fe3+ or O2 as an electron acceptor for anaerobic and aerobic respiration, respectively. S0, NO3−, NO2−, SO42−, Mn4+, fumarate, or Fe2O3 cannot serve as electron acceptors for anaerobic respiration. Growth occurs at 60–90°C and pH 1.0–5.0. The cells are nearly regular cocci, approximately 0.5–0.8 μm in diameter. The cell envelope consists of an S-layer attached to the cytoplasmic membrane and contains caldariellaquinone. The G + C content of the DNA is 29.9 mol%. The type strain is Acidianus manzaensis strain NA-1T, deposited in the culture collections of the National Institute of Technology and Evolution (NBRC 100595).
Literature Cited
Brierley CL, Brierley JA (1973) A chemoautotrophic and thermophilic microorganism isolated from an acid hot spring. Can J Microbiol 19:183–188
Caccavo F Jr, Lonergan DJ, Lovley DR, Davis M, Stolz JF, McInerney MJ (1994) Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol 60(10):3752–3759
Collins MD (1985) Analysis of isoprenoid quinones. Methods Microbiol 18:329–366
Dempsey GP, Bullivant S (1976) A copper block method for freezing non-cryoprotected tissue to produce ice-crystal-free regions for electron microscopy. J Microsc 106:251–260
Edward FD (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89:5685–5689
Fuchs T, Huber H, Burggraf S, Stetter KO (1996) 16S rDNA-based phylogeny of the archeal order Sulfolobus and reclassification of Desulfurolobus ambivalens as Acidianus ambivalens comb. nov. Syst Appl Microbiol 19:56–60
He ZG, Zhong H, Li Y (2004) Acidianus tengchongensis sp. nov., a new species of acidothermophilic archaeon isolated from an acidothermal spring. Curr Microbiol 48(2):159–163
Kashefi K, Holmes DE, Reysenbach AL, Lovley DR (2002) Use of Fe(III) as an electron acceptor to recover previously uncultured hyperthermophiles: isolation and characterization of Geothermobacterium ferrireducens gen. nov., sp. nov. Appl Environ Microbiol 68(4):1735–1742
Kashefi K, Lovley DR (2000) Reduction of Fe(III), Mn(IV), and toxic metals at 100 degrees C by Pyrobaculum islandicum. Appl Environ Microbiol 66(3):1050–1056
Kashefi K, Lovley DR (2003) Extending the upper temperature limit for life. Science 301(5635):934
Kashefi K, Tor JM, Holmes DE, Gaw Van Praagh CV, Reysenbach AL, Lovley DR (2002) Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int J Syst Evol Microbiol 52(3):719–728
Katayama-Fujimura Y, Komatsu Y, Kuraishi H, Kaneko T. (1984) Estimation of DNA base composition by high performance chromatography of its nuclease P1 hydrolysis. Agric Biol Chem 48:3169–3172
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Lovley DR, Holmes DH, Nevin KP (2004) Diddimilatory Fe(III) and Mn(IV) reduction. Adv Microbial Physiol 49:219–286
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil Trans R Soc Lond B Biol Sci. 358:59–83
Myers CR, Myers JM (1994) Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J Appl Bacteriol 76(3):253–258
Ohmura N, Sasaki K, Matsumoto N, Saiki H (2002) Anaerobic respiration using Fe3+, S0, and H2 in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. J Bacteriol 184(8):2081–2087
Segerer A, Neuner A, Kristjansson JK, Stetter KO (1986) Acidianus infernus gen. nov., sp. nov., and Acidianus brierleyi comb. nov.: facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria. Int J Syst Bacteriol 36:559–564
Thompson JC, Gibson TJ, Plewniak F, Leanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tool. Nucleic Acids Res 25:4876–4882
Author information
Authors and Affiliations
Corresponding author
Additional information
Strain NA-1 has been deposited in the culture collections of the National Institute of Technology and Evolution (NBRC 100595) and American Type Culture Collection (ATCC BAA 1057). The 16S rDNA sequence has been deposited at GenBank under accession number AB182498.
Rights and permissions
About this article
Cite this article
Yoshida, N., Nakasato, M., Ohmura, N. et al. Acidianus manzaensis sp. nov., a Novel Thermoacidophilic Archaeon Growing Autotrophically by the Oxidation of H2 with the Reduction of Fe3+. Curr Microbiol 53, 406–411 (2006). https://doi.org/10.1007/s00284-006-0151-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0151-1