Abstract
Xylanase production from B. megaterium was enhanced using solid state fermentation with respect to the use of solid substrate, moistening solution, moisture content, inoculum, sugars, soyabean meal, amino acids, and extraction with surfactant. An increase of ≈423-fold in xylanase production and complete suppression of CMCase production was achieved over submerged liquid fermentation. Biobleaching using this cellulase-free xylanase, 8 U/g of oven dried pulp of 10% consistency, showed 8.12% and 1.16% increase in brightness and viscosity, 13.67% decrease in kappa number, and 31% decrease in chlorine consumption at the CD stage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Xylanases have attracted considerable research interest because of their potential application in the biobleaching of pulp and other industrial systems [1]. Viikari et al. [2] first reported that xylanases decrease the use of chlorine needed for bleaching kraft pulp and play an important role when the use of hazardous chemicals is to be decreased in bleaching processes. Many researchers have confirmed and extended this observation, and the technology is now being commercialized [3, 4].
In the paper production process, pulping is a step during which cellulose fibers are broken apart and most of the lignin is removed. The remaining lignin is then removed by a multistep bleaching process [4]. Pulping and bleaching are both performed at high temperatures. Hence, the paper industry needs xylanases that are thermostable and, preferably, active at neutral and alkaline pH [4]. Also, xylanases are mostly contaminated with cellulases, which destroy the structure of cellulose and diminish pulp quality. This means xylanases with a high degree of cellulase-free purity are required. So the application of an alkali- and heat-stable cellulase-free xylanase for large-scale pulp bleaching biotechnology requires efforts that are aimed at process optimization, simplification, and cost reduction. The production of xylanases must, therefore, be improved by finding potent fungal or bacterial strains or by inducing mutant strains to produce and excrete greater amounts of enzymes or by enhancing production by solid state fermentation (SSF). SSF is a well-adapted and cheaper process than submerged liquid fermentation (SLF) and the amounts of products obtained by SSF are many-fold higher. In addition, the products obtained have slightly different properties (e.g., more thermotolerance). Besides, low-moisture content reduces the possibilities of contamination by bacteria and yeast. Higher levels of aeration, simple culture media that provide all the nutrients necessary for growth, simple design reactors with few spatial requirements that can be used due to the concentrated nature of the substrates and low energetic requirements (mechanical agitation and aeration are not necessary), are other advantages [5]. There are several reports on xylanase production by SSF using fungi, but few on alkaline xylanase productions by SSF using bacteria [6, 7]. Bacteria are preferred over fungi as they are a good source of alkaline and thermostable enzymes. SSF by bacteria are primarily confined to Bacillus spp., which could be attributed to their ability to adhere to the substrate particles to produce filamentous cells for penetration and to their specific need for water activity [8]. The present work reports improvement in culture conditions and medium composition so as to obtain high xylanase yields from B. megaterium and its application in biobleaching.
Materials and Methods
Microbial Strain
An alkalophilic Bacillus strain was isolated from soil/water samples, collected from the vicinity of a paper mill in Mohali (Punjab), on seed M162 agar plates, pH 8, containing 0.2% xylan. The microscopic, morphological, physiological, and biochemical tests were performed according to Bergey’s Manual of Systematic Bacteriology [9].
Xylanase Production
Submerged liquid fermentation (SLF).
Xylanase production was carried out in 25 ml of MS2 medium, inoculated with 1–2% of overnight inoculum, made in the same medium, and incubated for 24 h at 37°C and 200 rpm. Enzyme was obtained after centrifugation at 10,000g for 10 min.
Solid state fermentation (SSF)
Five grams of solid substrate, moistened with mineral salt solution, was inoculated and incubated at 37°C in an incubator humidified by keeping a tray of sterile distilled water (relative humidity 60–70%). The contents were shaken intermittently. The enzyme from each flask was extracted with 50 ml of distilled water. The whole content was squeezed through wet muslin cloth. The extract was centrifuged at 10,000g for 30 min and the supernatant was used as enzyme source. Enzyme productions were done at least in triplicates and average values and standard errors were calculated.
Conditions for the Optimization of Xylanase Production in SSF
-
1.
Moistening solutions: Various mineral salt solutions and tap water were used. (Table 1)
-
2.
Moisture level: Different substrate to moistening solution ratios (1:1, 1:1.5, 1:2, 1:2.5) were used.
-
3.
Soya bean meal: 1–5 % was added as a nitrogen source in the medium.
-
4.
Inoculum size and age: An 8–24-h-old culture at 1–20% size was used as inoculum.
-
5.
Incubation period: SSF was carried out for 24–168 h.
-
6.
Sugars: Xylose, glucose, lactose, mannose, arabinose, and maltose were added at 1%.
-
7.
Polysaccharides: Xylan, soluble starch, chitin, and guar gum were added at 1%.
-
8.
Amino acids: Glycine, isoleucine, proline, leucine, alanine, valine, serine, threonine, and cysteine were added at 0.2%.
-
9.
Solid substrates: Wheat bran, rice bran, and oil cakes were used.
-
10.
Extraction: Xylanase was extracted with distilled water and 0.01, 0.02, 0.04, 0.07, and 0.1% concentrations of Tween 80.
Enzyme Assays
Xylanase and CMCase activities were determined by the dinitrosalicylic acid (DNSA) method [10, 11]. One unit of xylanase (CMCase) activity was defined as the amount of enzyme that released 1 μmol of reducing sugar equivalent to xylose (glucose) per ml per minute. Xylanase production was expressed as units/g of solid substrate.
Scale Up of Solid State Fermentation
Enamel metallic trays of sizes 35 × 25 × 5 and 40 × 30 × 5 cm3 containing 250 and 500 g of solid substrate, respectively, were moistened with suitable moistening solutions and used to cultivate the bacterial strains after covering with aluminium foil and autoclaving. The wet solid bacterial cultures were extracted and assayed for xylanase and CMCase.
Biobleaching of Kraft Pulps (ECDED1D2)
Pulp used for biobleaching was hardwood pulp obtained from Ballarpur Industries Limited (BILT), Yamunanagar, Haryana, India, and was a mixture obtained from woods of six different trees, namely poplar, eucalyptus, eucalyptus rulla, small vaneer, bamboo, and debarka bamboo hardwood (DBH). Extensively washed pulp (50 g) of 10% consistency was treated with xylanase (8 U/g pulp) in a plastic bag under optimum conditions. Xylanase-treated pulp was bleached with chemical sequence CDED1D2 (Table 2). Reduction in chlorine consumption to obtain same amount of brightness in both xylanase-treated and control (non-treated) was seen by conducting an experiment in which all the parameters and treatment conditions were kept the same except for the dose of chlorine treatment given in the CD stage.
Analytical Techniques
Kappa number, a measure of lignin content, was determined by reaction of pulp samples with acidified potassium permanganate (Tappi method T 236). Viscosity, which indicates cellulose chain length, was determined by dissolving delignified pulp in cupriethylenediamine (CED) and measuring the viscosity of a 0.5% solution with an Ostwald viscometer (Tappi method T 230). Brightness of a paper sheet was measured as % ISO with ISO (TechniBright, USA). % ISO (International Organisation of Standardisation) brightness is the measure of diffused reflectance at 457 nm. Total organic chlorine (TOCl) was determined by the method of Hong et al. [12].
Results and Discussion
Microbial Strain
The isolated strain was a gram-positive, spore (central, ellipsoidal) -forming rod (2.2–2.5 × 0.4–0.6). It grew optimally at 40°C (range 20–50°C), pH 8.0 (range 5–10.5) and in the presence of 0.02% sodium azide and 10% sodium chloride, which were inhibitory for most other Bacillus strains. The microscopic, morphological, physiological, and biochemical characters of the isolate revealed that it belonged to genus Bacillus and on the basis of similarity coefficients (SJ) measured pair wise with B. brevis (0.437), B. cereus (0.57), B. coagulans (0.53), B. circulans (0.53), B. megaterium (0.85), and B. stearothermophilus (0.43), using 25 characters, the identity of the isolate was found closest to B. megaterium. This isolate was used for the optimization of the following parameters of SSF.
Moistening Solutions
Xylanase production was supported maximally by MS9 (96.8 U/g) followed by MS8 (90 U/g) and minimally by MS1 (52 U/g). It appears that high phosphate content (amount or buffering) of MS9 and MS8 may be important.
Moisture Level
Xylanase titers were highest at 1:1.5 (105 U/g) and lowest at 1:2.5 (73 U/g). The importance of moisture level in SSF media and its influence on microbial growth and product biosynthesis may be attributed to the impact of moisture on the physical properties of the solid substrate [13]. A higher than optimum moisture level causes decreased porosity, alteration in wheat bran particle structure, gummy texture, lower oxygen transfer, and enhancement of the formation of aerial mycelia [13, 14]. Likewise, a lower than optimum moisture level leads to reduced solubility of the nutrients of the solid substrate, lower degree of swelling, and higher water tension.
Soya Bean Meal
Five percent concentration increased xylanase production maximally by 1.4-fold (130 U/g).
Inoculum Size and Age
Sixteen-hour-old inoculum at 10% size yieded maximum xylanase production (400 U/g).
Incubation Period
Maximum production (415.5 U/g) was achieved after 96 h and was constant thereafter.
Sugars
The objective was to devise a medium that will enhance the production of xylanase and inhibit the production of CMCase. This happened in the presence of xylose (1%), as it resulted in an increase in xylanase production (460 U/g) and a decrease in CMCase production (from 20 to 4 U/g). Lactose (390 U/g), maltose (450 U/g), mannose (430 U/g), and arabinose (385 U/g) also supported good xylanase production. Maltose was not selected as it also enhanced CMCase production to 40 U/g. CMCase production by B. megaterium was completely repressed on the addition of 1% glucose to the medium though there was a slight (5%) decrease in xylanase production also. Bataillon et al. [15] and Beg et al. [16] reported that xylanase production was inducible in nature and a high yield of xylanase was obtained when xylose was supplemented in the medium. Catabolite repression on the addition of xylose to the medium was also reported in many Bacillus spp. [15, 17]. There are reports of bacterial xylanase production, resistant to repression by glucose and xylose [18].
Polysaccharides
The presence of xylan, soluble starch, chitin, and guar gum at 1% concentration in the medium decreased xylanase production by 8, 9, 27, and 10%, respectively. A strong inducing effect of xylan on xylanase production has been reported [19]. The induction of xylanase biosynthesis by xylan occurs via the low molecular weight soluble catabolites, which are generated from xylan by the action of constitutively produced extracellular or cell surface located xylan degrading enzymes [20].
Amino Acids
Among all the amino acids tried, the maximum stimulatory effect was seen in the medium supplemented with hydrophobic compared to hydrophilic amino acids. Among the hydrophobic group, glycine exhibited the highest (31.14%) increase in yields to 465 U/g followed by isoleucine, proline, and leucine producing 420, 410, and 370 U/g, respectively. Alanine and valine are also hydrophobic but decreased xylanase production slightly by 0.14 and 0.15%, respectively. Among the hydrophilic amino acids, only serine resulted in 0.11% increase while threonine and cysteine decreased xylanase production by 0.12 and 0.24%, respectively. Balakrishnan et al. [21] reported a 2.5-fold enhancement in xylanase production by Bacillus sp. NCL 87-6-10 using glycine and casamino acids. The combination of DL-norleucine, L-leucine, and DL-isoleucine stimulated endoxylanase production from Streptomyces sp. QG-11-3 by 6.72-fold [22].
Solid Substrates
Wheat bran supported maximum xylanase production (650 U/g) compared to rice bran (94 U/g), wheat + rice bran (120 U/g), and wheat bran + oil cakes (200 U/g). The universal suitability of wheat bran may be because it contains sufficient nutrients and is able to remain loose even in moist conditions, thereby providing a large surface area [23]. However, use of other solid substrates like grain byproducts, cassava, potato, beans, and sugar beet pulp have also been reported [24]. dos Santos et al. [25] used sugarcane bagasse for xylanase production by Thermoascus aurantiacus.
Extraction
Maximum extraction was achieved with 0.01% Tween 80 (746 U/g) compared to 540 U/g with distilled water.
Final SSF Conditions
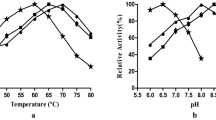
Ten percent of 16-h-old culture was used as inoculum for 5 g wheat bran moistened (1:1.5) with MS 9 containing 1% xylose, 1% glucose, 0.2% glycine, and 5% soyabean meal and incubated at 37°C for 96 h for xylanase production. Enzyme extraction was done with Tween 80 (0.01%). The final yields of xylanase and CMCase were 846 and 0 U/g, respectively (Fig. 1).
Scale Up of SSF
Scale up from 5 g in flasks to 500 g in enamel trays resulted in a slight decrease (12.14%) in xylanase production. Xylanase production in enamel trays was comparable with that in culture flasks and scaling up did not result in a reduction of xylanase titers in another study [6].
Biobleaching of Kraft Pulp
The kraft pulp was pretreated with xylanase. Pulp brightness and viscosity increased by 8.12% (7 brightness points) and 1.16%, respectively, and kappa number reduced by 13.67% (2.26 points) (Table 3) as compared to control (not treated with xylanase). Also, prebleaching with xylanase from B. megaterium caused 31.25% reduction in chlorine consumption at CD stage (Table 4). Xylanase from Thermatoga maritima increased the brightness of oxygen-delignified hardwood kraft by 3.8% ISO [26]. Xylanase P (a commercial xylanase from Sappi forest Products, Southern Africa) improved the brightness of kraft pulp by 5.6 brightness points when used at 7 U/g of moisture-free pulp and caused an approximately 10% reduction in chlorine dioxide consumption [27].
The SSF conditions optimized in this work have resulted in ∼423-fold increase in xylanase yield compared to SLF. Also, the CMCase has been completely suppressed under these conditions, thus circumventing the need to find cellulase-negative isolates or mutants. The slight increase in the viscosity of xylanase-treated pulp showed the absence of CMCase. Optimized SSF thus serves as an alternative to the generation of xylanase hyperproducing recombinants. The xylanase preparation has shown potential in bleaching the pulp and reducing the consumption of chlorine dioxide significantly. This would, in turn, result in the reduction of absorbable organic halogens and chloride levels of the bleach wastewaters and alleviate the environmental impact of the industry.
Literature Cited
Kuhad RC, Singh A (1993) Lignocellulosic biotechnology: current and future prospects. Crit Rev Biotechnol 13:151–172
Viikari L, Ranua M, Kantelinen A, Sundquist J, Linko M (1986) Bleaching with enzymes. Proc 3rd Int Conf Biotechnology Pulp and Paper Industry, Stockholm, 16–19:67–69
Rättö M, Mathrani IM, Ahring B, Viikari L (1994) Application of thermostable xylanase of Dictyoglomus sp. in enzymatic treatment of kraft pulps. Appl Microbiol Biotechnol 41:130–139
Srinivasan MC, Rele MV (1999) Microbial xylanases for paper industry. Curr Sci 77:137–142
Raimbault M, (1998) General and microbiological aspects of solid substrate fermentation. Electronic J Biotechnol 1(3):1–15
Archana A, Satyanarayana T (1997) Xylanase production by thermophillic Bacillus licheniformis A99 in solid state fermentation. Enzyme Microb Technol 21:12–17
Gessesse A, Mamo G (1999) High-level xylanase production by an alkalophilic Bacillus sp. by using solid state fermentation. Enzyme Microb Technol 25:68–72
Satyanarayana T (1994) Production of bacterial extracellular enzymes by solid state fermentation. In: Solid State Fermentation, Pandey A (ed), New Delhi: Wiley Eastern Ltd., pp 122–129
Claus D, Berkley RCW (1986) Genus Bacillus. In: Sneath HA, Mair NS, Sharpe ME (eds) Bergey’s manual of systemic bacteriology, vol. 2. Baltimore: Williams and Williams, pp 1104–1139
Bailey MJ (1992) Interlaboratory testing off methods for assay of xylanase activity. J Biotechnol 23:257–270
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31(1):426–428
Hong Q, Shin NH, Chang H (1989) Effect of oxygen extraction on organic chlorine contents in bleach plant effluents. Tappi J 72(6):157–161
Raimbault M, Alazard D (1980) Culture method to study fungal growth in solid fermentation. Eur J Appl Microbiol Biotechnol 9:199–209
Feniksova RV, Tikhomrova AS, Rakhleeva BE (1960) Conditions for forming amylase and proteinase in surface culture of Bacillus subtilis. Mikrobiolgica 29:745–748
Bataillon M, Cardinali APN, Duchiron F (1998) Production of xylanases from a newly isolated alkalophilic thermophilic Bacillus sp. Biotechnol Lett 20:1067–1071
Beg QK, Bhushan B, Kapoor M, Hoondal GS (2000a) Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3. J Ind Microbiol Biotechnol 24:396–402
Rajaram S, Varma A (1990) Production and characterization of xylanase from Bacillus thermoalkalophilus grown on agricultural wastes. Appl Microbiol Biotechnol 34:141–144
Srivastava KC (1993) Properties of thermostable hemicellulolytic enzymes from Thermomonospora strain 29 grown in solid state fermentation on coffee processing solid waste. Biotechnol Adv 11(3):441–465
Sunna A, Prowe SG, Stoffregen T, Antranikian G (1997) Characterization of the xylanases from the new isolated thermophilic xylan-degrading Bacillus thermoleovorans strain K-3d and Bacillus flavothermus strain LB3A. FEMS Microbiol Lett 148:209–216
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Balakrishnan H Srinivasan MC, Rele MV (1997) Extracellular protease activities in relation to xylanase secretion in an alkalophilic Bacillus sp. Biotechnol Lett 18:599–601
Beg QK, Bhushan B, Kapoor M, Hoondal GS (2000b) Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalyptus kraft pulp. Enzyme Microb Technol 27:459–466
Babu KR, Satyanarayana T (1995) α-Amylase production by thermophilic Bacillus coagulans in solid state fermentation. Proc Biochem 30:305–309
Pandey A (1992) Recent process development in solid state fermentation. Proc Biochem 27:1–8
dos Santos E, Piovan T, Roberto IC, Milagres AM (2003) Kinetics of the solid state fermentation of sugarcane bagasse by Thermoascus aurantiacus for the production of xylanase. Biotechnol Lett 25(1):13–6
Shah AK, Cooper D (2000) Adolphson R and Eriksson K-EL. Xylanase treatment of oxygen-bleached hardwood kraft pulp at high temperature and alkaline pH levels gives substantial savings in bleaching chemical. J Pulp Pap Sci 26:8–11
Madlala AM, Bisson S, Singh S, Christov L (2001) Xylanase induced reduction of chlorine dioxide consumption during elemental chlorine-free bleaching of different pulp types. Biotechnol Lett 23:345–351
Degryse E, Glandroff L, Pierard A (1978) A comperative analysis of extreme thermophillic bacteria belonging to genus Thermus. Arch Microbiol 117:186–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sindhu, I., Chhibber, S., Capalash, N. et al. Production of Cellulase-Free Xylanase from Bacillus megaterium by Solid State Fermentation for Biobleaching of Pulp. Curr Microbiol 53, 167–172 (2006). https://doi.org/10.1007/s00284-006-0051-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0051-4