Abstract
The development of rhizobial inoculants requires the selection of isolates that are symbiotically efficient as well as adapted to the local environmental conditions. Our aim was to find indigenous chickpea rhizobia tolerant to adverse environmental conditions, such as temperature and pH. Thirteen isolates of chickpea mesorhizobia from southern Portugal were examined. Tolerance to stress temperatures and pH was evaluated by quantification of bacterial growth at 20–37°C and pH 5–9, respectively. Tolerance to heat shocks was studied by submitting isolates to 46°C and 60°C. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis protein analysis revealed qualitative and quantitative differences when isolates were submitted to temperature stress. A 60-kDa protein was overproduced by all isolates under heat stress. Almost all isolates revealed to be more tolerant to 20°C than to 37°C. A positive correlation was found between the maximum growth pH and the isolate origin soil pH. Generally, isolates more tolerant to temperature stress showed a lower symbiotic efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rhizobia are soil bacteria able to infect the roots of leguminous plants, promoting the formation of nitrogen-fixing nodules. The symbiosis between rhizobia and legumes is the most important biological mechanism for providing nitrogen to the soil/plant system, thus reducing the need of plant crops for chemical fertilizers [6, 45]. Nitrogen fixation depends on the physiological state of the host plant [45] and on the effectiveness and environmental fitness of the microsymbiont [39], as well as on the interaction between the two partners. For example, chickpea (Cicer arietinum L.) is a successful legume on alkaline soils [35], whereas the symbiosis between rhizobia and chickpea is better adapted to acidity [12, 38].
Several important steps in the symbiosis process, like nodule formation and nitrogen fixation, are affected by stress conditions, which might be considered limiting factors [45]. Incubation of cultures at high temperatures often results in loss of nodulation properties and nitrogen-fixation ability in Sinorhizobium meliloti and Rhizobium trifolii [41, 46]. Studies with cowpea nodules show that nitrogenase activity was not affected with increasing temperature from 15°C to 35°C, but decreased severely at temperatures above 38°C [34].
pH stress also limits nodulation and nitrogen fixation [9]. Soil acidity constrains symbiotic nitrogen fixation, limiting rhizobial survival and persistence in soils and affecting the exchange of molecular signals between rhizobia and their host, thus reducing nodulation [10, 13]. However, when either the microsymbiont or the host plant is acid tolerant, nodulation and plant development are similar to that achieved when both symbiotic partners are acid tolerant [42].
The heat shock response consists in the cell’s adaptation to a temperature increase, characterized by an overproduction of a set of proteins, termed heat shock proteins (HSPs), which are important to survival during stress conditions [24, 25, 27, 28, 32]. Among these are molecular chaperones [14] that play an important role in protein folding [7]. The chaperonins, ring-shaped chaperones, such as Hsp60 [7], have an essential function in promoting protein folding under stress conditions [11]. Several studies have focused on HSPs found in rhizobia [23, 44, 45], but none have been reported on chickpea rhizobia.
Previous studies indicate a correlation between strain performance under in vitro stress in pure culture and strain behavior under stress conditions [15]. Thus, in vitro evaluation of strains under stress might be a useful method in finding rhizobial isolates adapted to different environments, where extreme temperatures and pH limit symbiotic nitrogen fixation.
In the present study, the effect of stress temperatures and pH was evaluated on indigenous chickpea mesorhizobia from different regions in southern Portugal: Beja, Elvas-Casas Velhas (CV), Elvas-Estação Nacional de Melhoramento de Plantas (ENMP), Évora, and Setúbal. As far as we know, this is the first report on the analysis of protein profiles from chickpea rhizobia, submitted to temperature stress.
Materials and Methods
Bacterial isolates
Twelve rhizobial isolates were previously obtained from Beja, Elvas-CV, Elvas-ENMP, and Évora soils [18, 19]. The isolate from Setúbal soil was obtained from root nodules of chickpea trap plants, as described by Vincent [43]. All sampling sites are located within a region of about 100 km2 in the South of Portugal [20]. The isolates used in this study and soil pH of their origin site are listed in Table 1. The indigenous rhizobia isolates used in this study were identified as Mesorhizobium by 16S rDNA sequence analysis [20]. Isolates were maintained in tryptone yeast (TY) [1] agar slants at 4°C and in TY broth containing 20% (v/v) glycerol at –80°C. The Mesorhizobium mediterraneum-type strain UPM-Ca36T was used as reference [31].
Effect of temperature and pH stress on rhizobia growth
Tolerance of isolates to heat and cold stress was evaluated by quantification of bacterial growth (optical density at 540 nm) in liquid Yeast Extract Mannitol (YEM) medium [43] at temperatures of 37°C and 20°C, respectively, after 48 h (stationary phase). Tolerance of isolates to extreme pH values was evaluated simultaneously in medium with pH of 5 and 9. The control conditions were considered to be 28°C and pH 7 [2].
Effect of heat shocks on rhizobia growth
Tolerance to heat shock was evaluated by quantification of bacterial growth, after a shock at 60°C for 15 min or 46°C for 3 h, followed by 48 h growth, at 28 °C, pH 7.
Analysis of protein patterns
Changes in the protein patterns induced by temperature stress and heat shocks were detected by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis [17] of total cell proteins. Cell treatment and electrophoresis were done as described by Laranjo et al. [20].
Statistical analysis
In order to compare tolerance of different isolates to distinct conditions, the optical density obtained for each isolate after 48 h at 28°C, pH 7 was considered as 100% growth. Data represent the average of three replicates per treatment. Spearman correlations were performed.
Results and Discussion
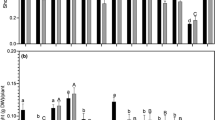
As expected for rhizobia [39], most isolates grew better at 28°C. However, isolates 92-Évora, 98-Évora, and EE-29-ENMP showed their maximum growth at conditions different from the control (28°C, pH 7) (Fig. 1). Isolate 98-Évora revealed a maximum growth at 20°C, an unexpected result because the optimal temperature range described for rhizobial growth is 25–30°C [39]. The maximum temperature for growth of chickpea rhizobia is reported to be 40°C, both for M. ciceri and M. mediterraneum [30, 31]. Contrary to these results, Maâtallah et al. [21, 22] described a maximum temperature range of 30–35°C for chickpea rhizobia.
Isolates grew more efficiently at 20°C than at 37°C, except isolates 78-Elvas and 83-Elvas, which were more tolerant to 37°C. Isolate EE-13-ENMP was the most tolerant to both temperature stresses (20°C and 37°C), showing about 90% growth at 20°C, pH 7, and about 60% growth at 37°C, pH 7 (Fig. 1).
No significant correlation was found between tolerance of isolates to heat stress and the maximum air temperature of their origin site (Table 1). However, the isolates most tolerant to 37°C (78-Elvas, 83-Elvas and EE-13-ENMP) were from the two regions with the highest maximum air temperature (Elvas-CV and Elvas-ENMP).
Analysis of tolerance to heat shocks revealed that several isolates endure the 60°C shock better than the 46°C shock (Fig. 2). These isolates include 78-Elvas, EE-12-ENMP, and EE-13-ENMP, EE-12-ENMP being the most tolerant one, with 63% growth compared to the control. Other isolates, such as 92-Évora, 98-Évora, and EE-29-ENMP, are more resistant to the 46°C shock, particularly 98-Évora. The strain Ca36T and isolates ST-2-Setúbal, 27-Beja, 83-Elvas and 85-Elvas revealed a low tolerance to heat shock, as their growth was reduced to 2–14%.
Relative to the heat shock at 60°C, isolates from Elvas-ENMP were the most resistant ones. This result suggests a relationship between isolate tolerance to heat shocks and the maximum air temperature of its geographical origin (Table 1). No relationship was found between tolerance to high-temperature stress and tolerance to heat shocks. For instance, isolate EE-12-ENMP is resistant to heat shocks, but grows poorly at 37°C (20% growth). On the other hand, isolate 78-Elvas was tolerant at 37°C (90% growth), but sensitive to heat shocks.
Generally, isolates found to be more tolerant to temperature stress showed lower symbiotic efficiencies [19]. The isolate EE-13-ENMP, which showed tolerance to 37°C, has a low symbiotic efficiency (25.3%) [19]. On the other hand, isolate 27-Beja, with a higher symbiotic efficiency (33.9%) [19], revealed a low tolerance to 37°C and to heat shocks. Similarly, La Favre and Eaglesham [16] were unable to find a clear correlation between a strain’s capacity to grow at high incubation temperatures and its ability to nodulate under high-temperature stress. However, Munevar and Wollum [26] found a correlation between the ability of a rhizobial isolate to nodulate and its capacity to grow at high incubation temperatures.
Although the optimal pH range described for rhizobial growth is 6–7 [39], some isolates showed a higher growth in medium with pH values different from 7. For instance, with isolate 92-Évora, maximum growth was obtained at pH 5, and isolate EE-29-ENMP showed maximum growth at pH 9. Furthermore, some isolates showed changes in the preferential pH of culture medium with the temperature. For example, at 28°C, isolates 27-Beja and 31-Beja grew more at pH 7, but at 20°C, the growth rate was higher at pH 9.
Our isolates’ pH range tolerance is in agreement with Nour et al. [29], in which chickpea-infective strains are more tolerant than rhizobia in general, with a pH tolerance range of 4.5–10. However, other authors report a pH range of 5–8 [21, 22]. A positive correlation (r = 0.5; P < 0.01) was depicted between maximum growth pH and the origin soil pH of isolates (Table 1). Isolates from Beja, Elvas-ENMP, and Setúbal (alkaline soils) showed major growth at neutral or alkaline pH. On the other hand, isolates from Elvas-CV and Évora (acid soils) revealed higher growth at pH 5 or 7. Although Priefer and co-workers [33] mentioned the absence of correlation between bean rhizobia tolerance to different pH values and the origin soil pH, our results are in agreement with two previous reports on tree rhizobia [15, 40]. Based on the results concerning the effect of temperature stress on rhizobial growth, six isolates were chosen for SDS-PAGE analysis of the protein profiles generated at different growth temperatures, namely strain Ca36T and isolates 27-Beja, 85-Elvas, 98-Évora, EE-12-ENMP, and ST-2-Setúbal. SDS-PAGE analysis of isolates generated reproducible protein profiles.
Qualitative and quantitative differences in the protein patterns of isolates were detected after growth at 20°C and 37°C, when compared with the control conditions (28°C) (Fig. 3). However, the detected changes are distinct, depending on the isolate and growth conditions.
Analysis by SDS-PAGE of protein patterns of isolates 98-Évora, EE-12-ENMP (A), and isolates 27-Beja, ST-2-Setúbal, UPM-Ca36T, and 85-Elvas (B) grown under normal conditions (28°C) and under thermal stress (20°C and 37°C). Proteins mentioned in Table 2 are marked with an arrow.
In different isolates, proteins with the same molecular weight were overproduced under stress temperature. For example, isolates 98-Évora, EE-12-ENMP, and ST-2-Setúbal revealed the simultaneous overproduction of four proteins (67, 60, 57, and 24 kDa) when submitted to 37°C (Table 2). Furthermore, overproduction of proteins with the same molecular weight (such as 60, 34, or 32 kDa) was observed with some isolates grown at both stress temperatures (20°C and 37°C), which supports their important cellular functions.
Several isolates revealed proteins that were only detected after submitting the isolates to temperature stress (Table 2). For instance, with isolate 27-Beja, proteins of 53 kDa and 85 kDa were detected only after growth at 20°C. After growth at 37 °C, isolate EE-12-ENMP showed a 75-kDa protein. It is possible that the proteins detected only after the growth at 20°C or 37°C were synthesised de novo, suggesting their importance in the survival and growth of rhizobia in stress conditions. High tolerance to temperature stress (20°C or 37°C) was not apparently related with the number of overproduced proteins. For example, at 20°C, isolate 27-Beja revealed the overproduction of 10 proteins, whereas with Ca36T, only 2 proteins were overproduced. However, under those conditions, the growth of isolate 27-Beja was 38%, whereas growth of strain Ca36T was 65%. Studies with arctic and temperate isolates of rhizobia isolated from Astragalus and Oxytropis revealed that several HSPs were induced when cells were shifted from their optimal growth temperature to shock temperatures [3].
The molecular weight values of the proteins that were overproduced after the growth at 37°C were different from the values reported in other studies with rhizobia [3, 23, 44]. Previous studies [44] revealed that rhizobia isolates isolated from Acacia senegal and Prosopis chilensis submitted to temperature stress promoted the production of a protein of 65 kDa. Our results show that a protein of 60 kDa was overproduced in all indigenous isolates at 37°C and in isolates 27-Beja and 85-Elvas at 20°C. Cloutier et al. [3] detected the overproduction of a protein with a similar molecular weight (59.5 kDa) for all heat shock treatments tested (29–46.4°C), which apparently did not confer a greater tolerance to temperature stress. Our results lead to similar conclusion, because isolates showed different tolerances to 37°C, in spite of the presence of the 60-kDa protein in all of them (Table 2).
The molecular weight value of the protein detected in the present study (60 kDa) as well as the observation that it is overproduced upon stress conditions might suggest its identification as the heat shock protein GroEL [37]. This protein is involved in nif gene regulation in Bradyrhizobium japonicum [4] and Klebsiella pneumoniae [8]. It is also required as a chaperonin for the assembly of a functional nitrogenase [5, 8, 36], at least in Bradyrhizobium japonicum.
With heat shocks, SDS-PAGE analysis did not reveal any new bands. Isolates revealed only slight differences in the protein patterns by comparison with the control conditions (data not shown); a few proteins were overexpressed or underexpressed in some isolates, agreeing with other studies with temperate and arctic rhizobia [3]. Isolate 27-Beja showed a decrease in the expression of a 43-kDa protein after the shocks of 46°C and 60°C. Isolate ST-2-Setúbal revealed a small increase in the synthesis of a 70-kDa protein after the 60°C shock and the absence of a 85-kDa protein after the 46°C shock.
The development of rhizobial inoculants requires the selection of isolates that are symbiotically efficient and tolerant to adverse environmental conditions. Here, we report the analysis of tolerance to temperature and pH stress of Portuguese mesorhizobia isolates able to nodulate chickpea. The analysis of total protein profiles after temperature stress showed significant differences in the protein patterns, and further characterisation of the overproduced proteins might help to clarify the mechanisms involved in stress tolerance.
Literature Cited
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Cadahía E, Leyva A, Ruiz-Argüeso T (1986) Indigenous plasmids and cultural characteristics of rhizobia nodulating chickpeas (Cicer arietinum L.). Arch Microbiol 146:239–244
Cloutier J, Prevost D, Nadeau P, Antoun H (1992) Heat and cold shock protein synthesis in arctic and temperate strains of rhizobia. Appl Environ Microbiol 58:2846–2853
Fischer HM, Babst M, Kaspar T, Acuña G, Arigoni F, Hennecke H (1993) One member of a groESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J 12:2901–2912
Fischer HM, Schneider K, Babst M, Hennecke H (1999) GroEL chaperonins are required for the formation of a functional nitrogenase in Bradyrhizobium japonicum. Arch Microbiol 171:279–289
Freiberg C, Fellay R, Bairoch A, Broughton WJ, Rosenthal A, Perret X (1997) Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394–401
Frydman J (2001) Folding of newly translated proteins in vivo: The role of molecular chaperones. Annu Rev Biochem 70:603–647
Govezensky D, Greener T, Segal G, Zamir A (1991) Involvement of GroEL in nif gene regulation and nitrogenase assembly. J Bacteriol 173:6339–6346
Graham PH (1992) Stress tolerance in Rhizobium and Bradyrhizobium, and nodulation under adverse soil-conditions. Can J Microbiol 38:475–484
Graham PH, Draeger KJ, Ferrey ML, Conroy MJ, Hammer BE, Martinez E, Aarons SR, Quinto C (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici Umr1899. Can J Microbiol 40:198–207
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Howieson JG, O’ Hara GW, Carr SJ (2000) Changing roles for legumes in Mediterranean agriculture: Developments from an Australian perspective. Field Crop. Res. 65:107–122
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop Res. 65:151–164
Jakob U, Gaestel M, Engel K, Buchner J (1993) Small heat shock proteins are molecular chaperones. J Biol Chem 268:1517–1520
Kulkarni S, Nautiyal CS (1999) Characterization of high temperature-tolerant rhizobia isolated from Prosopis juliflora grown in alkaline soil. J Gen Appl Microbiol. 45:213–220
La Favre AK, Eaglesham ARJ (1986) The Effects of high-temperatures on soybean nodulation and growth with different strains of Bradyrhizobia. Can J Microbiol 32:22–27
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laranjo M, Rodrigues R, Alho L, Oliveira S (2001) Rhizobia of chickpea from southern Portugal: Symbiotic efficiency and genetic diversity. J Appl Microbiol 90:662–667
Laranjo M, Branco C, Soares R, Alho L, Carvalho MD, Oliveira S (2002) Comparison of chickpea rhizobia isolates from diverse Portuguese natural populations based on symbiotic effectiveness and DNA fingerprint. J Appl Microbiol 92:1043–1050
Laranjo M, Machado J, Young JPW, Oliveira S (2004) High diversity of chickpea Mesorhizobium species isolated in a Portuguese agricultural region. FEMS Microbiol. Ecol. 48:101–107
Maâtallah J, Berraho E, Sanjuan J, Lluch C (2002) Phenotypic characterization of rhizobia isolated from chickpea (Cicer arietinum) growing in Moroccan soils. Agronomie 22:321–329
Maâtallah J, Berraho EB, Muñoz S, Sanjuan J, Lluch C (2002) Phenotypic and molecular characterization of chickpea rhizobia isolated from different areas of Morocco. J Appl Microbiol 93:531–540
Michiels J, Verreth C, Vanderleyden J (1994) Effects of temperature stress on bean-nodulating Rhizobium strains. Appl Environ Microbiol 60:1206–1212
Münchbach M, Dainese P, Staudenmann W, Narberhaus F, James P (1999) Proteome analysis of heat shock protein expression in Bradyrhizobium japonicum. Eur J Biochem 264:39–48
Münchbach M, Nocker A, Narberhaus F (1999) Multiple small heat shock proteins in rhizobia. J Bacteriol 181:83–90
Munevar F, Wollum AG (1982) Response of soybean plants to high root temperature as affected by plant cultivar and Rhizobium strain. Agron J 74:138–142
Narberhaus F, Weiglhofer W, Fischer HM, Hennecke H (1996) The Bradyrhizobium japonicum rpoH1 gene encoding a sigma 32-like protein is part of a unique heat shock gene cluster together with groESL1 and three small heat shock genes. J Bacteriol 178:5337-5346
Nocker A, Hausherr T, Balsiger S, Krstulovic N-P, Hennecke H, Narberhaus F (2001) A mRNA-based thermosensor controls expression of rhizobial heat shock genes. Nucl Acids Res 29:4800–4807
Nour SM, Cleyet-Marel JC, Beck D, Effosse A, Fernandez MP (1994) Genotypic and phenotypic diversity of Rhizobium isolated from chickpea (Cicer arietinum L.). Can J Microbiol 40:345–354
Nour SM, Fernandez MP, Normand P, Cleyet-Marel J-C (1994) Rhizobium ciceri sp. nov., consisting of strains that nodulate chickpeas (Cicer arietinum L.). Int J Syst Bacteriol 44:511–522
Nour SM, Cleyet-Marel J-C, Normand P, Fernandez MP (1995) Genomic heterogeneity of strains nodulating chickpeas (Cicer arietinum L.) and description of Rhizobium mediterraneum sp. nov. Int J Syst Bacteriol 45:640–648
O’Connell KP, Gustafson AM, Lehmann MD, Thomashow MF (2000) Identification of cold shock gene loci in Sinorhizobium meliloti by using a luxAB reporter transposon. Appl Environ Microbiol 66:401–405
Priefer UB, Aurag J, Boesten B, Bouhmouch I, Defez R, Filali-Maltouf A, Miklis M, Moawad H, Mouhsine B, Prell J, Schlüter A, Senatore B (2001) Characterisation of Phaseolus symbionts isolated from Mediterranean soils and analysis of genetic factors related to pH tolerance. J Biotechnol 91:223–236
Rainbird RM, Atkins CA, Pate JS (1983) Effect of temperature on nitrogenase functioning in cowpea nodules. Plant Physiol 73:392–394
Rao DLN, Giller KE, Yeo AR, Flowers TJ (2002) The effects of salinity and sodicity upon nodulation and nitrogen fixation in chickpea (Cicer arietinum). Ann Bot 89:563–570
Ribbe MW, Burgess BK (2001) The chaperone GroEL is required for the final assembly of the molybdenum-iron protein of nitrogenase. Proc Natl Acad Sci USA 98:5521–5525
Rusanganwa E, Singh B, Gupta RS (1992) Cloning of Hsp60 (GroEL) peron from Clostridium perfringens using a polymerase chain-reaction based approach. Biochim Biophys Acta 1130:90–94
Siddique KHM, Loss SP, Regan KL, Jettner RL (1999) Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust J Agric Res 50:375–387
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia. New York: Springer-Verlag
Surange S, Wollum AG, Kumar N, Nautiyal CS (1997) Characterization of Rhizobium from root nodules of leguminous trees growing in alkaline soils. Can J Microbiol 43:891–894
Toro N, Olivares J (1986) Analysis of Rhizobium meliloti Sym mutants obtained by heat-treatment. Appl Environ Microbiol 51:1148–1150
Vargas AAT, Graham PH (1988) Phaseolus vulgaris cultivar and Rhizobium strain variation in acid-ph tolerance and nodulation under acid conditions. Field Crop Res 19:91–101
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Oxford: Blackwell Scientific
Zahran HH, Rasanen LA, Karsisto M, Lindström K (1994) Alteration of lipopolysaccharide and protein profiles in SDS-PAGE of rhizobia by osmotic and heat-stress. World J Microbiol Biotechnol 10:100–105
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Zurkowski W (1982) Molecular mechanism for loss of nodulation properties of Rhizobium trifolii. J Bacteriol 150:999–1007
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (POCTI/BME/44140/2002). M. Laranjo acknowledges a PhD fellowship (SFRH/BD/6772/2001) from Fundação para a Ciência e a Tecnologia. We thank G. Mariano for technical assistance with the isolate collection and I. Duarte for air temperature values at ENMP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, C.S., Laranjo, M. & Oliveira, S. Effect of Heat and pH Stress in the Growth of Chickpea Mesorhizobia. Curr Microbiol 53, 1–7 (2006). https://doi.org/10.1007/s00284-005-4515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-4515-8