Abstract
Trichoderma species are readily isolated from Brazilian cerrado soil by conventional methods and some of them were characterized as Trichoderma koningii. The effect of carbon source on the production of β-1,3-glucanases in the culture filtrates of a specific Trichoderma koningii strain (ALL 13) was investigated. Enzyme activity was detected in all carbon sources tested and only one band of β-1,3-glucanase was detected in non-denaturing PAGE. This enzyme was purified by Sephacryl S-200 gel filtration and Phenyl Sepharose CL 4B chromatography. A typical procedure provided 105-fold purification with 13.4% yield. The molecular weight of the purified enzyme was 75 kDa as estimated by SDS-PAGE. The enzyme hydrolyzed laminarin in an endo-like fashion to form small oligosaccharides and glucose. The Km and Vmax values for β-1,3-glucanase, using laminarin as substrate, were 0.148 mg.mL−1 and 0.159 U.min−1, respectively. The pH optimum for the enzyme was pH 4.6 and maximum activity was obtained at 50°C. Hg2+ inhibited the purified enzyme.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Trichoderma species are readily isolated from Brazilian cerrado soil by conventional methods and used for technological exploitation for enzyme production and biological control [10]. The application of combined molecular data, morphology, physiology, and colony characteristics showed that some of the strains isolated were identified as Trichoderma koningii. This strain showed that it was able to produce high amounts of β-1,3-glucanase, an enzyme system capable of degrading fungal cell walls [5, 10]. β-1,3-glucan is a cell-wall component of most fungi and β-1,3-glucanases have been found to be directly involved in the mycoparasitism interaction between Trichoderma species and its host [5]. These enzymes can hydrolyze the substrate by two possible mechanisms: (1) exo-β-1,3-glucanases (EC 3.2.1.58) hydrolyze β-glucans by sequentially cleaving glucose residues from the nonreducing end, and (2) endo-β-1,3-glucanases (EC 3.2.1.39) cleave β-linkages at random sites along the polysaccharide chain, releasing smaller oligosaccharides and glucose [18]. However, synergistic action between at least two enzymes with different modes of action is common in fungi that degrade β-glucans.
A considerable amount of research has been aimed at elucidating the β-1,3-glucanase system of Trichoderma species, mainly T. harzianum, during growth in different carbon sources [7]. It has been found to be a complex system consisting of at least three distinct isoenzymes, as detected by polyacrylamide or isoelectrofocusing gels. Some of these β-1,3-glucanases were recently purified from the supernatants of T. harzianum grown in minimal medium, supplemented with chitin as carbon source [7]. These enzymes are distinguishable on the basis of differences in physicochemical properties and molecular weight (29 to 76 kDa). However, little is known about the regulation of the expression of these enzymes [4]. This report describes the characteristics of a β-1,3-glucanase produced by Trichoderma koningii, and compares their properties with those produced by T. harzianum.
Materials and Methods
Organism and culture conditions
Spores from Trichoderma koningii (ALL13), isolated from the cerrado soil of the central region of Brazil (Enzymology Group collection, UFG/ICB), were collected in sterile saline, centrifuged at 2000 rpm, washed twice, and used as inoculum (1.0 × 107 spores ml−1) in liquid medium (TLE). TLE medium contained: 0.1% bactopeptone, 0.03% urea, KH2PO4 0.2%, 1.4% (NH4)2SO4, 0.03% MgSO4.7H2O, 0.03% glucose, and 0.1% (v/v) trace elements solution containing Fe2+, Zn2+, Mn2+, Cu2+ [14]. Purified cell wall from Rhizoctonia solani (0.5%), chitosan (0.5%), chitin (0.5%), starch (0.5%), and glucose (1.0%) were used as carbon source. The cultures were grown in conical flasks with constant shaking (140 rpm) at 28°C for 96 hours. The mycelium was harvested by filtration through filter paper, and the culture filtrate was dialyzed overnight against distilled water, freeze-dried, and used as source of β-1,3-glucanases. Purification of cell wall from R. solani was made by the method described by Mitchell and Taylor [13].
β-1,3-glucanase assay
Enzyme activity was measured by mixing 50 μl of sample with 100 μl of 50 mM acetate buffer (pH 5.0), containing 0.25% laminarin (Sigma). The mixture was incubated at 40°C for 30 min and the reducing sugar produced was determined by the method described by Miller [12]. One unit (U) of β-1,3-glucanase activity was defined as the amount of enzyme that produced 1 μmol of reducing sugar min−1 under the above conditions. Protein concentration was determined by the method of Lowry [11], using bovine serum albumin as standard.
Enzyme purification
The concentrated samples were loaded on a Sephacryl S-200 column (2.5 × 85 cm) previously equilibrated with 50 mM sodium acetate buffer, pH 5.0, and eluted at a flow rate of 40 ml h−1. Fractions of 2.0 mL were collected and monitored for protein (A280) and β-1,3-glucanase activity. Fractions containing β-1,3-glucanase activity were pooled and applied directly onto a Phenyl-Sepharose CL4B column (1.0 × 10 cm) equilibrated with 50 mM sodium acetate buffer, pH 5.0, containing 0.5 mM ammonium sulfate and eluted at a flow rate of 30 ml h−1. The column was washed with the same buffer and eluted with a linear gradient of 0.5-0 M ammonium sulfate. Fractions containing β-1,3-glucanase activity were pooled, dialyzed against water, and stored at −20°C.
Electrophoresis and enzymatic activities in gel
Polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine protein purity and the molecular mass of the purified enzyme under denaturing conditions using a 12% acrylamide gel, as described by Laemmli [8]. Protein was silver stained as described by Blum et al. [1]. Molecular weight markers were as follows: myosin (200 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (29 kDa).
Enzymatic activities in the gel were carried out as described by Pan et al. [17]. After non-denaturing PAGE, gels were washed with distilled water, incubated with 50 mM sodium acetate (pH 5.0) for 60 min, and then incubated at 40°C for 180 min in a solution containing 0.5% of laminarin (in 50 mM sodium acetate, pH 5.0). Bands with β-1,3-glucanase activity were located after boiling the gel with 2,3,5-triphenyltetrazolium chloride solution (TTC).
Enzyme characterization
The effect of pH on the enzyme activity was determined by varying the pH of the reaction mixtures using 100 mM sodium citrate (pH 2.3–4.0), 100 mM sodium acetate (pH 4.2–5.4), and 100 mM sodium phosphate (pH 5.8–7.1). The effect of temperature on the enzymatic activity was determined at pH 5.0, in the range 25 to 70°C. The effects of metallic ions and some enzyme inhibitors on β-1,3-glucanase activity were determined after preincubation at 50°C for 5 min. Michaelis-Menten constant (Km) was determined from Lineweaver-Burk representation of data obtained by measuring the initial rate of laminarin hydrolysis using a range of 0.1 to 1.0 mg ml−1.
Analysis of hydrolysis products
The purified β-1,3-glucanase was incubated with 1.0 mg ml−1 laminarin in 100 mM sodium acetate buffer (pH 5.0) at 40°C. Samples were removed after 2, 6, and 24 h incubation and hydrolysis was stopped by heating the samples in boiling water for 10 min. The products were detected by thin-layer chromatography (TLC), as described by Lato et al. [9]. Glucose and laminarobiose (dimer of glucose]) are used as standard.
Results and Discussion
β-1,3-glucanase production
The effects of different carbon sources on β-1,3-glucanase production by T. koningii (ALL13) were tested in TM medium supplemented with cell walls purified from Rhizoctonia solani (CWRS), chitin, chitosan, cellulose, starch, and glucose (Fig. 1A). Cultures were grown for 96 h, harvested and analyzed for β-1,3-glucanase activity. The fungus produced β-1,3-glucanases in all carbon sources, but the highest activity was obtained when CWRS were used as inducer (Fig. 1A). However, significant levels of β-1,3-glucanase activity were also found in presence of chitin, cellulose, chitosan, glucose, and starch. High levels of β-1,3-glucanase activity in the culture containing CWRS suggests that the regulation of this enzyme in T. koningii (ALL13) was also influenced by the inducer as described by other strains of T. harzianum [2, 4, 15]. To determine which secreted protein corresponded to β-1,3-glucanases, we assayed for enzyme activity by performing non-denaturing PAGE. Only one band with β-1,3-glucanase activity was detected in all carbon sources used (Fig. 1B). Multiple forms of these enzymes have been detected in strains of T. harzianum, and in some cases the syntheses are regulated differently. Production of three β-1,3-glucanases by T. harzianum TC [15], four β-1,3-glucanases by T. harzianum CECT2413 [2], five β-1,3-glucanases by T. harzianum T-Y [19], and seven β-1,3-glucanases by T. harzianum IMI206040 [20] has been described.
(A) Effect of carbon source on the production of β-1,3-glucanase from T. koningii. (B) Detection of extracellular β-1,3-glucanase activity on non-denaturing PAGE. Carbon sources (0.5%) used were as follows: (1) chitosan; (2) CWRS; (3) cellulose; (4) glucose; (5) starch; (6) chitin. Ten micrograms of protein was applied in each well. All values are the means of three replicates; standard deviations (SD) were less than 10% of mean.
Most of the β-1,3-glucanases produced by different isolates of T. harzianum are repressed by high concentrations of glucose [2, 14, 15, 20]. Consequently, it was of interest to study the effect of glucose on the β-1,3-glucanase produced by T. koningii (ALL13) (Fig. 2A and B). High activity was detected in the medium containing CWRS as a carbon source (Fig. 2A). Addition of glucose (1%) in this medium decreased the enzyme activity and also the band with β-1,3-glucanase activity in the non-denaturing PAGE (Fig. 2B). These results suggested that the synthesis of this enzyme from T. koningii (ALL13) is also controlled by carbon catabolic repression.
(A) Effect of glucose on the production of β-1,3-glucanase from T. koningii. (B) Detection of extracellular β-1,3-glucanase activity on non-denaturing PAGE. Carbon sources (0.5%) used were as follows: (1) glucose (0.5%); (2) glucose (1.0%); (3) glucose (1.0%) + CWRS (0.5%); (4) CWRS (0.5%). Ten micrograms of protein was applied in each well. All values are the means of three replicates; standard deviations (SD) were less than 10% of mean.
β-1,3-glucanase purification
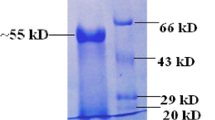
The β-1,3-glucanase produced by T. koningii (ALL13) in CWRS- containing medium was purified by using two-step procedures: gel filtration on Sephacryl S-200 and hydrophobic interaction on Phenyl Sepharose CL 4B. The enzyme was purified 105-fold with a recovery of 13.4% (Table 1). SDS-PAGE showed that the enzyme migrated as a single band with an estimated molecular mass of 75 kDa (Fig. 3 and Table 2). The molecular weights of β-1,3-glucanases produced by Trichoderma appear to vary considerably, not only between organisms, but also within the same species. Molecular masses of β-1,3-glucanases from T. harzianum T-Y [19] and T 24 [4] were in a similar range of 75, while smaller 29, 31, and 40 kDa β-1,3-glucanase also have been isolated and characterized [6, 14, 16].
β-1,3-glucanase characterization
The purified enzyme appeared to act in an endo-glucanase-like fashion, as indicated by the releasing of glucose and oligosaccharides after 6- and 24-hour incubation with laminarin (Fig. 4). Endo -β-1,3-glucanases are enzymes that hydrolyze the β-glucan chain by sequentially cleaving oligosaccharides and glucose residues from the nonreducing end of laminarin [18]. These enzymes have been described in many fungi, including those produced by T. harzianum [3, 6, 16].
The apparent Km (0.148 mg ml−1) of the endo-β-1,3-glucanases from T. koningii (ALL13) was substantially lower than that reported for T. harzianum (2.1 mg ml−1) [16], T. harzianum TC (1.72 mg ml−1) [6], T. harzianum CECT 2413 (3.3 mg ml−1) [2], and was almost similar to those reported for T. harzianum TY (0.1 mg ml−1) [19].
The effect of pH and temperature on the enzyme activity with laminarin as substrate was examined (Table 2). The optimal pH for the enzyme activity (4.6) was similar to that found for exo- and endo-β-1,3-glucanases from a variety of T. harzianum strains [3, 6, 7, 14, 16]. Generally, the β-1,3-glucanases from Trichoderma have optimum pH in an acid range of 4.0 and 6.2. The optimum temperature was found to be 50°C measured at pH 4.6. The enzyme retained 50% of maximum activity at 45°C and totally lost the activity at 45°C. β-1,3-glucanases from Trichoderma, in general, show high activity in the temperature range of 40–60°C and the stability decreased drastically after 60°C. The Hg2+ strongly inhibited the enzyme activity. The complete inhibition by mercuric ions may indicate the importance of indole amino acids in enzyme function, as has been demonstrated for an exo- and endo-β-1,3-glucanase from T. harzianum TC [3, 6, 14, 16].
In conclusion, T. koningii produces only one protein with β-1,3-glucanase activity when induced with cell wall isolated from R. solani. A 75-kDa β-1,3-glucanase was purified and exhibited high affinity for the substrate laminarin and was shown to be thermostable. However, further study will be required to determine if other enzymes are involved in the hydrolysis of cell walls from R. solani.
Literature Cited
Blum H, Beier H, Gross H (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
De La Cruz J, Pintor-Toro JA, Benitez T, Llobell A, Roero LA (1995) Novel endo-β-1,3-glucanase, BGN13.1, involved in the mycoparasitism of Trichoderma harzianum. J Bacteriol 177(23):6937–6945
Dubourdieu D, Desplanques C, Villettaz J, Ribereau-Gayon P (1985) Investigations of an industrial β-D-glucanase from Trichoderma harzianum. Carb Res 144:277–287
El-Katatny MH, Gudelj M, Robra kh, Elnaghy MA, Gubitz GM (2001) Characterization of a chitinase and an endo- β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl Microbiol Biotechnol 56:137–143
Harman GE (2000) Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis 84:377–393
Kitamoto Y, Kono R, Shimotori A, Mori N, Ichikawa Y (1987) Purification and some properties of an exo-β-1,3-glucanase from Trichoderma harzianum. Agric Biol Chem 51(12):3385–3386
Kononova GL, Markovich NA (2003) Lytic enzymes of Trichoderma and their role in plant defense from fungal disease: a review. Appl Biochem Microbiol 39:3341–3351
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature 227:680–685
Lato M, Brunelli B, Ciuffini G (1969) Thin layer chromatography of carbohydrates on silica gel impregnated with sodium acetate, monosodium phosphate and disodium phosphate. J Chromatog 39:407–417
Lima AL (2002) Molecular and biochemical characterization of Trichoderma isolated from Brazilian cerrado soil. PhD Thesis. Universidade de Brasília, Brasilia, Brazil
Lowry OH, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin Phenol Reagent. J Biol Chem 193:265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal Chem 31:426–428
Mitchell AD, Taylor IE (1969) Cell-wall proteins of Aspergillus niger and Chaetomium globosum. J Gen Microbiol 59:103–109
Noronha EF, Ulhoa CJ (1996) Purification and characterization of an endo-β-1,3-glucanase from Trichoderma harzianum. Can J Microbiol 42:1039–1044
Noronha EF, Kipnis A, Junqueira-Kipnis AP, Ulhoa CJ (2000) Regulation of a 36-KDa β-1,3-glucanase synthesis in Trichoderma harzianum. FEMS Microbiol Letters 188:19–22
Noronha EF, Ulhoa CJ (2000) Characterization of a 29-KDa β-1,3-glucanase from Trichoderma harzianum. FEMS Microbiol Lett 183:119–123
Pan SQ, Ye XS, Kue J (1989) Direct detection of β-1,3-glucanase isoenzymes on polyacrylamide electrophoresis and isoelectrofocusing Gels. Anal Biochem 182:136–140
Pitson SM, Seviour RJ, McDougall BM (1993) Noncellulolytic fungal β-glucanases: their physiology and regulation. Enz Microbiol Technol 15:178–192
Ramot O, Cohen-Kupiec R, Chet I (2000) Regulation of β-1,3-glucanase by carbon starvation in the mycoparasite Trichoderma harzianum. Mycol Res 104(4):415–420
Vazquez-Garciduenas S, Leal-Morales CA, Herrera-Estrella A (1998) Analysis of the β-1,3-glucanolytic system of the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 64(4):1442–1446
Acknowledgments
This work was supported by a biotechnology research grant to C.J.U. (CNPq and FUNAPE/UFG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monteiro, V.N., Ulhoa, C.J. Biochemical Characterization of a β-1,3-Glucanase from Trichoderma koningii Induced by Cell Wall of Rhizoctonia solani. Curr Microbiol 52, 92–96 (2006). https://doi.org/10.1007/s00284-005-0090-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-0090-2