Abstract
Oenococcus oeni has numerous amino acid requirements for growth and dipeptides could be important for its nutrition. In this paper the individual or combined effect of dipeptides on growth of O. oeni X2L in synthetic media deficient in one or more amino acids with L-malic acid was investigated. Utilization of dipeptides, glucose, and L-malic acid was also analyzed. Dipeptides were constituted by at least one essential amino acid for growth. Dipeptides containing two essential amino acids, except leucine, had a more favorable effect than free amino acids on the growth rate. Gly-Gly was consumed to a greater extent than Leu-Leu and a rapid exodus of glycine to the extracellular medium accompanied it. The microorganism could use glycine in exchange for other essential amino acids outside the cell, favoring growth. In the presence of Leu-Leu, the increase in glucose consumption rate could be related to the additional energy required for dipeptide uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Oenococcus oeni, the species of lactic acid bacteria (LAB) more frequently associated with malolactic fermentation (MLF), in wine has numerous nutritional requirements for growth [8, 9, 20]. Amoroso et al. [2] reported that four strains of O. oeni had an absolute requirement for a minimum of four amino acids for growth and in one of them (X2L strain) the number of essential amino acids significantly increased when L-malic acid was present in the medium. Certainly L-malic and citric acids are the substrates that LAB utilizes most frequently in wine. Saguir and Manca de Nadra [18] demonstrated that citric acid metabolism was involved in the biosynthesis of aspartate-derived amino acids by O. oeni. Considering the amino acid requirements of O. oeni strains, peptide utilization may have great nutritional value for growth. On the other hand, wine has a limited amino acid content [1, 4]. Feuillat et al. [6] found that the peptide fraction extracted from wine, especially peptides of molecular weight lower than 1000, was more favorable for the growth of O. oeni than the amino acid or protein fractions. Manca de Nadra et al. [12, 13] reported the proteolytic activity of O. oeni X2L on the nitrogenous macromolecular fraction of white and red wines, which favored peptide release.
The aim of this work was to describe the individual or joint effect of different dipeptides as sources of amino acids on the growth of O. oeni X2L in a synthetic medium supplemented with L-malic acid. At the same time the utilization of dipeptides, glucose, and L-malic acid was analyzed. The use of a chemically defined medium with L-malic acid and low amino acid concentration was necessary to evaluate the influence of dipeptides on the growth of O. oeni in conditions of nutritional stress, such as wine.
Materials and Methods
Microorganism
O. oeni X2L was isolated from an Argentinean red wine [14, 19]. The strain was stored at −20°C in MRS medium [5] supplemented with tomato juice (15%) and glycerol (30%, v/v).
Culture media, growth conditions, and culture procedures
A chemically defined medium [11] supplemented with L-malic acid (2.5 g/L) was used as basal medium (BM) and consisted of the following composition in distilled water (per liter): D-glucose, 10 g; potassium acetate, 10 g; potassium dihydrogen orthophosphate, 2 g; sodium thioglycollate, 0.5 g; magnesium sulphate.7H2O, 0.15 g; manganese sulphate.4H2O, 0.02 g; ferrous sulphate.7H2O, 0.01 g; Tween-80, 1 mg; and (in mg/L): adenine, 50; cytidylic acid, 50; deoxyguanosine, 50; guanine-HCl, 50; thymidine, 50; uracil, 50; p-aminobenzoic acid, 0.01; vitamin B12, 0.01; calcium pantothenate, 1; D-biotin, 0.01; folic acid, 0.1; niacin, 1; pyiridoxal ethyl acetal HCl, 0.5; riboflavin, 0.5; thiamine HCl, 1. Amino acid concentrations are given in Table 1. The modified BM with dipeptides (Sigma, St. Louis, MO) contained (in mmol/L): glycyl-glycine, 2.2 as a replacement for glycine; leucyl-leucine, 0.22 as a replacement for leucine; leucyl-proline, 0.45 or 0.34 as a replacement for leucine or proline respectively; prolyl-aspartic acid, 0.34 or 1.5 as a replacement for proline or aspartic acid respectively; and methionyl-proline, 0.34 or 0.33 as a replacement for proline or methionine respectively.
A semisynthetic medium where the amino acid source, except cysteine-HCl, was substituted by tryptone (4 g/l) was used for adaptation of the cells before their inoculation into the synthetic media. All media were adjusted to pH 4.8 with 1 N HCl before sterilization. The different synthetic media were sterilized in an autoclave, with heating stopped immediately on reaching 121°C. Cysteine-HCl and dipeptides sterilized by filtration through a nylon membrane (0.22 μm pore size, Millipore) were added to sterilized media.
Cultures were prepared by first growing them in MRS broth with 15% tomato juice, pH 4.8, incubated, without agitation, at 30°C (optimal conditions for O. oeni growth). The cells were harvested by centrifugation at the end of the exponential growth phase (34 h) and precultured three times under the same conditions in the semisynthetic adaptation medium before inoculation in synthetic media. After 72 h of incubation, the cells of the last preculture (third) were harvested by centrifugation, washed twice with sterile distilled water to avoid carry-over nutrients, and resuspended in sterile distilled water (OD620 = 0.90). Different synthetic media were inoculated at a concentration of 6.5 × 105 cfu/mL. All cultures were incubated at 30°C for 5 days and the samples were taken at various times during growth and stored frozen (−18°C) for subsequent chemical analysis.
Growth measurement
Bacterial growth was monitored by periodic spectrophotometric measurements at 620 nm using a Bausch and Lomb Spectronic-20 spectrophotometer during O. oeni X2L growth. At the same times the colony-forming units (cfu/mL) were determined by plating 0.1 mL of inoculated medium on MRS medium supplemented with 20.0 g/L of agar. From these data it was possible to calculate the average growth rates.
The amino acid requirements were estimated in the medium by omitting these amino acids one at a time. They were classified into three groups according to the extent of growth (A, Table 2) in each deficient medium. From 0 to 10% of growth the amino acid was considered as essential from 10% to 50% as stimulatory, and over 50% as non-essential [2].
Kinetics of cell growth
Growth experiments were repeated at least three times. Growth data of O. oeni X2L in synthetic media were modeled according to the Gompertz equation as modified by Zwietering et al. [23]:

where y is the log cfu/mL at the time t; k is the initial cell concentration as log cfu/mL; A represents the difference in cell concentration between inoculum and stationary phase; μmax is the maximum growth rate as Δ log cfu/mL/h; and λ is the length of the lag phase expressed in hours.
Analytical methods
Dipeptides and amino acids were analyzed by reverse-phase HPLC (RP-HPLC) using an ISCO liquid chromatograph (ISCO, Lincoln, NE). Samples were submitted to a pre-column derivatization with o-phthaldiladehyde (OPA). The reagent solution for derivatization consisted of 200 mg OPA in 9 mL methanol, 1 mL 0.4 M sodium borate pH 10, and 160 μL 2-mecaptoethanol (MCE). Solvents used for separation were: solvent A: methanol, 10 mM sodium phosphate buffer, pH 7.3 and tetrahydrofuran (19:80:1) and solvent B: methanol and 10 mM sodium phosphate buffer, pH 7.3 (80:20). Solvent gradient conditions were as follows: 6 min (0 B); 10 min (15% B); 4 min (30% B); 12 min (40% B); 16 min (80% B) and 5 min (0 B). All separations were performed on a Waters Nova-Pack C18 column (150 × 3.9 mm i.d., 60 Å, 4 μm) with a flow of 1.0 mL/min. The detection was by fluorescence using a model 121 fluorimeter (340 nm excitation filter and 425 nm emission filter). Samples were injected in triplicate onto the column, after being filtered through a 0.22 μm filter. Prior to RP-HPLC analysis, all samples were diluted with 0.4 borate buffer, pH 10. Standards of amino acids and dipeptides were used to determine the concentration of free amino acids and glycyl-glycine or leucyl-leucine respectively. The standard solutions were prepared by dissolving each amino acid or dipeptide in a 0.1 N HCl solution to reach a concentration of 2.5 μmol/mL. These solutions were stored at −18°C. Aliquots of 50, 100, 200, and 500 μL of these solutions were adjusted to 25 mL with borate buffer 0.4 M pH 10.
The pre-column derivatization and the column apparatus were at room temperature.
D-Glucose was measured by the glucose oxidase method (Kit from Wiener Laboratory, Rosario, Argentina). L-Malic acid was determined by an enzymatic method (Boehringer Kit, Mannheim, Germany).
Statistical analysis
The experimental data were analyzed by one-way analysis of variance test. Variable means showing statistical significance were compared using Tukey’s test (Minitab student R12). All statements of significance are based on the 0.05 level of probability [16].
Results
Effect of dipeptides on O. oeni X2L growth in synthetic medium
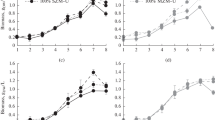
Figure 1 shows that in BM without L-malic acid O. oeni grew to a low cell density (5.53 × 106 cfu/mL). The L-malic acid incorporation markedly increased the growth rate and the population level about 3.4-fold. No growth was observed in the absence of glucose.
The O. oeni X2L growth parameters in BM following individual addition of Met-Pro, Leu-Pro, Pro-Asp, Gly-Gly, Leu-Leu or a dipeptide mixture containing Met-Pro, Leu-Pro, Gly-GIy and Leu-Leu in place of one or four amino acids respectively, are reported in Table 2. The strain did not grow or the growth was lower than 10% when leucine, methionine, proline or glycine were individually removed from BM, confirming that they are essential amino acids for growth. When leucine or proline were replaced by Leu-Leu or Leu-Pro respectively, the growth parameters were similar to those observed in BM. When Met-Pro or Pro-Asp were incorporated as the source of methionine or proline respectively, and Gly-GIy as a donor of glycine, the growth rate increased 26% but not the extent of growth. The removal of aspartic acid from BM reduced by 70% the growth rate and the extent of growth, confirming its stimulatory effect. When Pro-Asp was incorporated as the source of aspartic acid, the growth parameters were similar to those obtained in BM.
The addition of the dipeptide mixture in place of the respective free amino acids increased the growth rate 34%.
Dipeptide utilization by O. oeni X2L
Figure 2 shows the dipeptide utilization by O. oeni X2L in BM measured during bacterial growth at 24, 48, and 96 h of incubation. These experiments were carried out for Gly-Gly, which increased the growth rate, and for Leu-Leu, which did not modify growth parameters with respect to BM (Table 2). The utilization of Gly-Gly or Leu-Leu began immediately when growth began in BM deficient in glycine or leucine, respectively. Residual Gly-Gly or Leu-Leu concentrations decreased significantly at 48 h of incubation and represented 53.0% and 31.0% of the initial levels of Gly-Gly and Leu-Leu respectively. At the end of growth, Gly-Gly decreased by 1.16 mmol/L and Leu-Leu by 0.17 mmol/L, while the concentration of the hydrolysis dipeptide products increased by 0.48 mmol/L for glycine and 0.21 mmol/L for leucine. The fraction of glycine or leucine accumulated internally from dipeptides was 1.87 and 0.14 mmol/L, respectively.
The final consumption of free amino acids, shown in Table 3, was not significantly different to glycine or leucine accumulated internally from the respective dipeptide hydrolysis.
An interesting finding was the result shown in Table 4. Alanine utilization was 74% higher when Gly-Gly replaced glycine in the medium whilst no increase was observed when Leu-Leu replaced leucine in the medium.
Effect of dipeptides on glucose and L-malic acid utilization
O. oeni X2L consumed 12.8%, 23.5%, and 30.5% of the initial glucose concentration at 24, 48, and 96 h of incubation, respectively. L-Malic acid was co-metabolized with glucose during its growth and was completely utilized at the end of growth. Regarding glucose consumption at 24 h incubation, when Leu-Leu was added in place of leucine to BM, the amount of glucose utilization was 29% higher, even when no change was observed in the growth parameters. On the other hand when Gly-Gly was added in place of glycine, glucose utilization increased 63%, coinciding with the higher growth rate obtained in the presence of this dipeptide (data not shown).
No changes were observed in L-malic acid consumption when the dipeptides replaced free amino acids in BM.
Discussion
Saguir and Manca de Nadra [17] reported in O. oeni m strain, that the favorable effect of L-malic acid on bacterial growth results from an additional energy gain associated with L-malic acid decarboxylation.
When methionine, proline or glycine were replaced by Met-Pro, Pro-Asp, Gly-Gly or the dipeptide mixture containing Met-Pro and Gly-Gly in BM, O. oeni X2L grew at a higher growth rate. When aspartic acid was replaced by Pro-Asp no modification of the microorganism’s growth was observed. Taking account that methionine, proline, and glycine are essential amino acids for growth, the observation that dipeptides supplying two essential amino acids were more favorable than the free amino acids for growth is an important one. This could be related to the fact that dipeptides constituted by essential amino acids as the source of one of them, increase the concentration of the other present in the medium. For Leu-containing dipeptides (Leu-Leu or Leu-Pro) as the source of leucine, it was observed that the growth rate was not increased compared with that found in BM. This fact could be linked to a low utilization of leucine or Leu-containing dipeptides (Fig. 2). Foucaud et al. [7] reported that the growth response of L. lactis and Leuconostoc mesenteroides when dipeptides replaced the corresponding individual amino acid is dependent on the nature of the dipeptide.
Juillard et al. [10] emphasized the minor role of free amino acids relative to peptides during growth of Prt− L. lactis subsp. lactis in milk. By contrast Van Boven and Konings [22] reported for Lactococcus lactis subsp. cremoris E8 that μmax values obtained in chemically defined media with dipeptides were the same as in amino-acid-containing media. Aredes Fernández et al. [3] reported than in Pediococcus pentosaceus c1 isolated from Argentinean wine a similar dipeptide mixture in place of the respective amino acids decreased the growth parameters with respect to those observed with free amino acids, possibly due a limited amino acid uptake caused by a high concentration of peptides.
O. oeni X2L consumed mainly glycine or glycine-containing dipeptide rather than leucine or leucine-containing dipeptide in BM, and only a small fraction of leucine was accumulated internally from Leu-Leu. So this could be linked to a better incorporation of glycine in cell material. The high Gly-Gly utilization was accompanied by an increment in the glycine efflux to the extracellular environment. Such glycine efflux could be used by O. oeni X2L to exchange for other essential amino acids outside the cell, favoring its growth rate under poor nutritional conditions. Thus, alanine utilization was 74% higher when Gly-Gly replaced glycine in the medium whilst no increase was observed when Leu-Leu replaced leucine in the medium. Rice et al. [15] reported that in L. lactis, alanine, threonine, and glycine were capable of exchange with ‘‘pool’’ glycine.
In BM supplemented with Leu-Leu in place of leucine during the first hours of incubation the higher glucose consumption could be related to the additional energy required for dipeptide hydrolysis. According to Van Boven and Konings [21], the Leu-Leu hydrolysis by whole cells of L. lactis ssp. cremoris Wg2 was dependent on the presence of the energy source lactose.
In conclusion: (i) in general, essential amino acids containing dipeptides such as methionine, glycine, and proline, had a more favorable effect than the free amino acids on the O. oeni X2L growth rate but not on the extent of growth; (ii) Gly-Gly uptake was accompanied by a rapid exodus of amino acids to the extracellular medium; (iii) The higher Gly-Gly utilization significantly increased the glycine released outside the cell and the microorganism could employ it as an exchange mechanism for the incorporation of other amino acids such as alanine; (iv) glucose catabolism supplies energy for Leu-Leu uptake; and (v) L-malic acid catabolism that supplies additional energy for growth was not modified by incorporation of the individual or combined dipeptides.
Literature Cited
MA Amerine CS Ough (1980) Methods for analysis of must and wines Wiley New york
MJ Amoroso FM Saguir MC Manca de Nadra (1993) ArticleTitleVariation of nutritional requirements of Leuconostoc oenos by organic acids J Inter Sci Vigne Vin 27 135–144
PA Aredes Fernández FM Saguir MC Manca de Nadra (2003) ArticleTitleEffect of amino acids and peptides on growth of Pediococcus pentosaceus from wine Lat Am Appl Res 33 225–229
O Colagrande A Silva A Casoli (1984) ArticleTitleAcides aminés dans les vins mousseux Conn Vigne Vin 18 27–47 Occurrence Handle1:CAS:528:DyaL2cXksFeht78%3D
JC Man ParticleDe M Rogosa ME Sharpe (1960) ArticleTitleA medium for the cultivation of lactobacilli J Appl Bacteriol 23 130–135
M Feuillat P Bidan Y Rosier (1977) ArticleTitleCroissance des bactéries lactiques du vin a partir des principaux constituants azotes du vin Ann Technol Agricole 26 435–447 Occurrence Handle1:CAS:528:DyaE1cXlt1Kjtrc%3D
C Foucaud D Hemme M Desmazeaud (2001) ArticleTitlePeptide utilization by Lactococcus lactis and Leuconostoc mesenteroides J App Microbiol 32 20–25 Occurrence Handle10.1046/j.1472-765x.2001.00852.x Occurrence Handle1:CAS:528:DC%2BD3MXhtlKlsrw%3D
P Fourcassie E Makaga-Kabinda-Massard A Belardi A Maujean (1992) ArticleTitleGrowth, D-glucose utilization and malolactic fermentation by Leuconostoc oenos strains in 18 media deficient in one amino acid J Appl Bacteriol 73 489–496 Occurrence Handle1:CAS:528:DyaK3sXkslWhs70%3D
E Garvie (1967) ArticleTitleThe growth factor and amino acid requirements of the genus Leuconostoc, including Leuconostoc paramesentero (sp. nov.) and Leuconostoc oenos J Gen Microbiol 48 439–447 Occurrence Handle1:CAS:528:DyaF1cXhvFyjtw%3D%3D Occurrence Handle6052634
V Juillard D Le Bars ER Kunji WN Konings JC Gripon J Richard (1995) ArticleTitleOligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk Appl Environ Microbiol 61 3024–3030 Occurrence Handle1:CAS:528:DyaK2MXnt1aiurY%3D Occurrence Handle7487034
OV Ledesma A Ruiz Holgado G Oliver (1977) ArticleTitleA synthetic medium for comparative nutritional studies of lactobacilli J Appl Bacteriol 42 123–133 Occurrence Handle1:CAS:528:DyaE2sXhs1Ors70%3D Occurrence Handle853026
MC Manca de Nadra ME Farías MV Moreno-Arribas E Pueyo MC Polo (1997) ArticleTitleProteolytic activity of Leuconostoc oenos. Effect on proteins and polypeptides from white wine FEMS Microbiol Lett 150 135–139 Occurrence Handle10.1016/S0378-1097(97)00109-2 Occurrence Handle1:CAS:528:DyaK2sXjtl2rsLg%3D
MC Manca de Nadra ME Farías MV Moreno-Arribas E Pueyo MC Polo (1999) ArticleTitleA proteolytic effect of Oenococcus oeni on the nitrogenous macromolecular fraction of red wine FEMS Microbiol Lett 174 41–47 Occurrence Handle10.1016/S0378-1097(99)00119-6
MC Manca de Nadra AM Strasser de Saad (1987) ArticleTitleEvolution of lactic acid bacteria during the different stages of vinification of Cafayate (Argentine) wines Microbiol Alim Nutr 5 235–240 Occurrence Handle1:CAS:528:DyaL1cXit1Sht7c%3D
GH Rice FHC Stewart AJ Hillier JR Jago (1978) ArticleTitleThe uptake of amino acids and peptides by Streptococcus lactis J Dairy Res 45 93–107 Occurrence Handle1:CAS:528:DyaE1cXhs1KntLk%3D
AJ Rossman BL Chance (1998) Workshop statistics: discovery with data and Minitab Springer Berlin Heidelberg New York
FM Saguir MC Manca de Nadra (1998) ArticleTitleOrganic acids metabolism under different glucose concentrations of Leuconostoc oenos from wine J Appl Bacteriol 81 393–397
FM Saguir MC Manca de Nadra (2002) ArticleTitleEffect of L-malic and citric acids metabolism on the essential amino acid requirements for Oenococcus oeni growth J Appl Microbiol 93 295–301 Occurrence Handle10.1046/j.1365-2672.2002.01698.x Occurrence Handle1:CAS:528:DC%2BD38XmvVaqurY%3D Occurrence Handle12147078
AM Strasser de Saad MC Manca de Nadra (1987) ArticleTitleIsolation and identification of the lactic acid bacteria from Cafayate (Argentina) wines Microbiol Alim Nutr 5 45–49
RP Tracey JP Britz (1989) ArticleTitleThe effect of amino acids on malolactic fermentation by Leuconostoc oenos J Appl Bacteriol 67 589–595 Occurrence Handle1:CAS:528:DyaK3cXps12huw%3D%3D
A Boven ParticleVan WN Konings (1986) ArticleTitleEnergetics of Leucyl-Leucine hydrolysis in Streptococcus cremoris Wg2 Appl Environ Microbiol 51 95–100
A Boven ParticleVan WN Konings (1988) ArticleTitleUtilization of dipeptides by Lactococcus lactis ssp cremoris. Biochimie 70 535–542 Occurrence Handle10.1016/0300-9084(88)90090-9
MH Zwietering I Jongeberger FM Roumbouts K van’t Riet (1990) ArticleTitleModelling of bacterial growth curve Appl Environ Microbiol 56 1875–1881
Acknowledgment
This work was supported by grants from Consejo Nacional de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) and Fundación Antorchas, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández, P.A.A., Saguir, F.M. & de Nadra, M.C.M. Effect of Dipeptides on the Growth of Oenococcus oeni in Synthetic Medium Deprived of Amino Acids. Current Microbiology 49, 361–365 (2004). https://doi.org/10.1007/s00284-004-4367-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00284-004-4367-7