Abstract
The interleukin (IL)-17 cytokine family members IL-17A and IL-17F mediate inflammatory activities via the IL-17 receptor (IL-17R) complex, comprised of the IL-17RA and IL-17RC subunits. Proper regulation of the IL-17 signaling axis results in effective host defense against extracellular pathogens, while aberrant signaling can drive autoimmune pathology. Elucidating the molecular mechanisms underlying IL-17 signal transduction can yield an enhanced understanding of inflammatory immune processes and also create an avenue for therapeutic intervention in the treatment of IL-17-dependent diseases. To date, the fundamental signaling mechanisms used by the IL-17R complex are still incompletely defined. While current structure–function studies have primarily focused on the IL-17RA subunit, recent research indicates that the IL-17RC subunit plays a key role in modulating IL-17 responses. This review will examine what is known regarding IL-17RC function and provide a framework for future work on this subunit and its impact on human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytokines play an integral role in the coordination of the innate and adaptive arms of the immune system to achieve host defense. Cytokine release following immunological insult results in the activation of innate immunity to prime immune signaling networks, recruitment of effector cells to the site of infection, induction of site-specific host defense effector mechanisms, and initiation of adaptive immunity to promote pathogen clearance and specific immunologic memory. While there have been remarkable advances in understanding how individual branches of the immune system function, specific mechanisms by which these systems interface remain incompletely understood. The newly described interleukin (IL)-17 cytokine family, produced by a recently defined subset of CD4+ T helper (Th) cells known as “Th17”, has helped to bridge this gap, as Th17 cells connect the adaptive and innate immune responses [1]. This review centers on IL-17 signal transduction and, more specifically, a newly identified and essential subunit of the IL-17 receptor complex (IL-17RC).
Contrary to archetypal effectors of adaptive immunity, Th17 cells promote innate effector mechanisms of inflammation. The differentiation and priming of Th17 cells from naive CD4+ T cells requires a pro-inflammatory cytokine milieu, including IL-6, transforming growth factor-β and IL-1. In addition, the survival and stabilization of the Th17 subset requires IL-23, a pro-inflammatory cytokine closely related to IL-12 (reviewed in [2, 3]). Once fully differentiated and activated, Th17 cells secrete IL-17A, IL-17F, IL-21, IL-22, IL-26, and a number of chemokines. These cytokines regulate inflammatory responses by driving the production of innate mediators such as IL-6, CXC chemokines, antimicrobial factors, and acute phase proteins [4, 5]. Th17 cells play a major role at mucosal surfaces to promote inflammation, providing a beneficial function in an infection setting and deleterious one in an autoimmune context [6, 7]. The generation and function of IL-17-producing cells and the effects of their cognate cytokines have been reviewed extensively elsewhere [2, 3, 8, 9].
The IL-17 and IL-17 receptor family
Th17 effector function results from the IL-17 cytokine/IL-17 receptor signaling axis, which is strikingly different in sequence and structure from other cytokine families. The IL-17 cytokine family includes six structurally related isoforms: IL-17A, IL-17B, IL-17C, IL-17D, and IL-17F [10]. IL-17E, more commonly called IL-25, shares structural features with other IL-17 family members, though its biologic functions differ considerably [11, 12]. IL-17A and IL-17F (which are ∼55% homologous) are both produced by Th17 cells and are the best-characterized family members to date [13]. Both IL-17A and IL-17F function as homodimers, and recently, an IL-17A/F heterodimer was described [14–16]. The IL-17A homodimer was found to be the most potent IL-17 family effector molecule based on its induction of the target gene CXCL1, followed by the IL-17A/F heterodimer, and finally the IL-17F homodimer [15]. Additionally, both IL-17A and IL-17F have been shown to promote the inflammatory pathology of a number of autoimmune diseases such as rheumatoid arthritis, lupus, psoriasis, and asthma (discussed in detail in subsequent sections) [17, 18].
IL-17 cytokine family members induce downstream signaling through interactions with a specific cell surface receptor complex, but the nature of this receptor is poorly understood. The IL-17 receptor (IL-17R) subfamily includes IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE (reviewed in [10, 13]). IL-17RD is also known as similar expression to the fibroblast growth factor receptor (SEF), as it was initially described as having the same expression pattern as the fibroblast growth factor receptor during zebrafish development [19]. The best-characterized IL-17R molecules are the IL-17RA and IL-17RC subunits in part because of their interaction to form a receptor complex specific for IL-17A and IL-17F [20, 21] (see details below). Several studies and recent reviews have focused on IL-17RA and its signaling properties [22]. IL-17RC, however, remains relatively unexplored. This review focuses on what is currently known regarding IL-17RC and addresses its contribution to IL-17 signal transduction with reference to IL-17RA.
Overview of the IL-17 binding complex
Studies of IL-17RA have provided a framework for understanding the role of IL-17RC in IL-17A- and IL-17F-mediated signaling. Prior to the recognition that IL-17RC was an integral part of the IL-17R complex, fluorescence resonance energy transfer (FRET) studies of IL-17RA indicated that IL-17RA multimers exist on the cell surface in the absence of ligand [23, 24]. However, following binding of either IL-17A or IL-17F, a rapid conformational shift occurs resulting in a loss of FRET signal. This observation could be due to a complete dissociation of the IL-17RA subunits with each other, a large re-orientation of the cytoplasmic tails, and/or the recruitment of another unknown subunit or cytoplasmic signaling intermediate [23]. This report was followed by the discovery that IL-17RA co-immunoprecipitates with IL-17RC in a ligand-dependent manner, raising the possibility that the ligand-dependent loss of FRET between IL-17RA subunits results from oligomerization with IL-17RC [21]. Consistent with this, IL-17RC also forms large, multimeric complexes consistent with oligomerization with IL-17RA [25]. The precise stoichiometry of the IL-17R complex, either with or without ligand, still remains undefined.
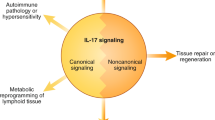
The IL-17R complex activates a number of different downstream effector signaling pathways. IL-17RA induces the activation of the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways to trigger many transcription factors, including the CCAAT/enhancer binding protein (C/EBP) transcription factors and AP1 (Fig. 1) [26–28]. Like most cytokine receptors, IL-17R family members do not contain intrinsic enzymatic activity but induce signaling via recruitment of signaling intermediates. The tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) adaptor serves as a molecular scaffold for a number of immune receptors, including the CD40 receptor (CD40R), the B cell-activating factor receptor (BAFFR), and IL-17RA [29, 30]. However, unlike the CD40R and the BAFFR, IL-17RA does not encode an obvious TRAF6-binding site. Rather, the adaptor Act1/CIKS links IL-17RA to TRAF6 [31, 32]. The N-terminus of Act1 contains a TRAF-binding domain while its C-terminus contains a SEF/IL-17R (SEFIR) domain, uniquely found on IL-17R family members [33]. Accordingly, Act1 plays a critical role in IL-17 signal transduction. Act1-deficient cells display severely blunted activation of IL-17 target genes such as IL-6 and CXC chemokines [31, 32]. Moreover, Act1−/− mice developed reduced inflammatory pathology in IL-17-dependent autoimmune encephalomyelitis and colitis models. However, IL-17RA signaling pathways independent of Act1 may also exist. In Act1−/− cells, extracellular signal-regulated kinases (ERK) are activated in response to IL-17 signaling, raising the possibility of novel IL-17RA signaling intermediates that warrant further investigation [31].
Alternative models of IL-17RC downstream signaling events. Two possible ways in which IL-17RC may participate in signaling are depicted. a Upon ligand binding, IL-17RC recruits a novel signaling intermediate, resulting in the following downstream effector functions: 1 possible posttranslational modifications on Act1, 2 recruitment of TRAF6, and/or 3 activation of novel effector signaling pathways. These events result in the creating of an appropriate molecular scaffold necessary for IL-17-dependent signaling. b Upon ligand binding, IL-17RC recruits Act1 through a homotypic SEFIR docking interaction. IL-17RC-bound Act1 oligomerizes with IL-17RA-bound Act1, resulting in an effective recruitment of intermediates for efficient IL-17-dependent signal transduction
As noted above, IL-17R family members encode a novel, conserved signaling motif termed SEFIR that bears homology to the Toll/IL-1R (TIR) signaling domain on the Toll-like/IL-1 receptors [34]. A BB-loop structure embedded within the TIR domain of the Toll-like receptors is critical for signaling, and IL-17RA contains a homologous motif termed the TIR-like loop (TILL) located downstream of the C-terminus of the IL-17RA SEFIR [35]. The TILL domain is vital for IL-17 signal transduction, as mutations specific to this region abrogate all IL-17-induced responses tested. Of note, the TILL appears to be unique to the IL-17RA SEFIR, which may explain why it is needed to partner with multiple IL-17R subunits [22]. The cytoplasmic tail of IL-17RC also contains the conserved SEFIR domain (Fig. 1) [34]. The IL-17RC SEFIR, however, has no obvious TILL structure, and thus far, no empirical studies have proven a functional role for the SEFIR in IL-17RC-dependent signaling.
Genomic structure and predicted splice variants of hIL-17RC and mIL-17RC. a hIL-17RC contains 19 exons. The transmembrane (TM) domain is encoded by exon 17 and spans amino acids 468–487. The SEFIR domain is encoded by exon 19 and spans amino acids 522–666. Exons with prime have multiple splice sites. Variants labeled with double dagger indicate that multiple variants within the exon were found. Variants labeled with asterisk have been independently verified [39, 41]. b mIL-17RC contains 18 exons. The TM domain is encoded by exon 17 and spans amino acids 465–484. The SEFIR domain is encoded by exon 18 and spans amino acids 495–645
The role of IL-17RC in mediating signaling is unclear. It is currently unknown whether Act1, TRAF6, or other signaling intermediates bind directly to IL-17RC. It is plausible that IL-17RC recruits novel adaptor intermediates to help create signaling scaffolds at the receptor complex, although none has been reported to date (Fig. 1a). Alternatively, IL-17RC may simply provide the necessary signaling domains to recruit an adequate number of signaling intermediates to promote effective signal transduction, analogous to the TNF receptor system (Fig. 1b) [36–38].
IL-17RC gene expression and regulation
Although the IL-17RA and IL-17RC subunits operate in concert to mediate IL-17 signaling, IL-17RC possesses a number of features that differentiate it from IL-17RA. Initially termed “IL-17R-like” (IL-17RL), IL-17RC was discovered via homology searches of mammalian expressed sequence tag (EST) databases and bears only 22% sequence homology with IL-17RA [39]. Alignment against the human genome indicates that the il17rc gene contains 19 exons on chromosome 3 and spans 16,550 bp within the chromosomal region 3p25.3 to 3.24.1 (Fig. 2). The murine il17rc gene contains 18 exons on chromosome 6 and spans 11,565 bp on the chromosomal arm 6q. The full-length human IL-17RC (hIL-17RC) contains 720 amino acids, and the murine IL-17RC (mIL-17RC) contains 698 amino acids. In both species, il17rc encodes a single pass type I transmembrane protein where the transmembrane domain is encoded in exon 17.
Intriguingly, the expression profile and tissue distribution of IL-17RC suggest that the gene regulation of IL-17RC differs considerably from IL-17RA. Specifically, epithelial cells of the prostate, kidney, and joints express high levels of IL-17RC mRNA, while low levels of expression are detected in the hematopoietic cell compartments [39–41]. Conversely, IL-17RA is highly expressed in the bone marrow, thymus, and spleen, but relatively low levels are detected in colon, small intestine, and lung [26, 40, 42]. The biological significance of this reciprocity in tissue expression is unknown but raises a number of interesting possibilities. IL-17RC or IL-17RA may bind a set of distinct ligands necessitating a different tissue distribution. Indeed, IL-17RA oligomerizes with IL-17RB to form a receptor complex that interacts with IL-25/IL-17E; accordingly, tissues sensitive to IL-25 may express higher levels of IL-17RA [43]. In addition, the differential gene regulation may be a mechanism to influence tissue specific signaling by IL-17A, IL-17F, and IL-17A/F because these ligands exhibit different binding affinities to the IL-17RC and IL-17RA subunits, as discussed in more detail below.
Unlike IL-17RA, which does not undergo alternative RNA splicing, IL-17RC exists in numerous splice forms. An EST database search and mRNA analysis of human prostate cancer cell lines revealed more than 90 different IL-17RC variants, some of which use cryptic splice sites (Fig. 2a) [39]. While most IL-17RC variants are spliced at sites in the extracellular domain, some mRNA transcripts suggest the existence of soluble receptors that could presumably influence IL-17 signaling [39]. A similar database search of mouse EST databases revealed only four mIL-17RC variants, none of which appears to be soluble (Fig. 2b) [41]. If they exist, soluble IL-17RC molecules could act as decoy receptors to dampen signaling, analogous to the IL-1 receptor antagonist (IL-1Ra) or receptor activator of NF-κB ligand (RANKL)/osteoprotegerin systems [44]. Alternatively, soluble IL-17RC could function analogously to the IL-6 receptor (IL-6R) system, in which a soluble IL-6Rα subunit promotes IL-6 binding to the gp130 receptor on cells that lack IL-6R, a process known as “trans-signaling” [45, 46]. Interestingly, mIL-17RC variants also appear to bind IL-17A and IL-17F with differing affinities, providing another level of potential signal modulation (Table 1). Moreover, some mIL-17RC variants bind neither of these two cytokines, hinting that there may be novel ligands or functions for IL-17RC (Table 1) [41].
Biological activity of IL-17RC
Investigations into the biological functions of IL-17RC have revealed a number of interesting observations. For example, there is a species-dependent cytokine binding and signaling role for IL-17RA and IL-17RC (Table 1). For example, hIL-17RA fails to reconstitute IL-17A- and IL-17F-dependent signaling in mIL-17RA−/− fibroblasts, whereas mIL-17RA rescues signaling. Only co-expression of hIL-17RC with hIL-17RA permits effective IL-17 signaling, suggesting that hIL-17RA cannot pair with the mIL-17RC endogenously expressed in murine cells [21]. This finding also indicates that, despite species distinctions, IL-17RC is required for IL-17 signal transduction in both systems [21, 41].
These studies also revealed that IL-17RC is not merely a ligand-binding component, but its cytoplasmic tail somehow contributes to signal transduction. Expression of hIL-17RA with an hIL-17RC lacking the cytoplasmic tail is not sufficient for IL-17-mediated induction of the CXCL1 target gene. The necessary portion of the IL-17RC tail needed for signaling is unknown, although the SEFIR domain is predicted to be important [34]. Consistent with these data, mIL-17RC−/− fibroblasts do not produce IL-6 or granulocyte-macrophage colony-stimulating factor in response to IL-17A or IL-17F stimulation, lending further support to the necessity of IL-17RC for signaling [47]. Thus far, IL-17RC-specific binding partners that interact with the cytoplasmic tail have not been identified.
The differences between the human and murine systems extend to IL-17A and IL-17F cytokine binding affinities. hIL-17RA binds preferentially to IL-17A and has a relatively low binding affinity to IL-17F. In contrast, hIL-17RC binds IL-17A and IL-17F with the same affinity [41]. In the murine system, the inverse is true: mIL-17RA binds IL-17A and IL-17F with equal affinities, but mIL-17RC binds preferentially to IL-17F. Therefore, in both humans and mice, IL-17RC appears to serve as a contact point for IL-17F (Table 1) [41]. The difference in IL-17RC binding affinities for IL-17A and IL-17F is of interest because this may serve as one mechanism to control and modulate IL-17 signaling. Although IL-17A and IL-17F have many overlapping effects including immunity to extracellular pathogens and neutrophil recruitment, their respective cytokine knockout models indicate that IL-17A plays a more significant role in driving autoimmunity [40, 48]. Moreover, in certain models of colitis, IL-17A plays a protective role while IL-17F has a pathogenic function [48–50]. These studies have also suggested that IL-17A can induce signals in murine T cells, which appear to lack IL-17RC, whereas IL-17F does not exert any T cell-specific effects [40, 42, 50]. This phenomenon raises the intriguing possibility that in T cells, IL-17RA homomultimers are sufficient for IL-17 signaling or that there exists a yet-to-be determined subunit that pairs with IL-17RA. It is also tempting to speculate that, given the tissue-specific expression level differences, there may exist receptor complexes with different ratios of IL-17RA and IL-17RC subunits in specific tissues, possibly explaining differential effects of IL-17A and IL-17F. Furthermore, the potential existence of agonistic soluble IL-17RC receptors could explain IL-17-dependent signaling in those tissues lacking IL-17RC expression, analogous to trans-signaling in the IL-6 system [45, 46]. Indeed, the differences in cytokine binding affinities and the central role IL-17RC possesses in the IL-17 signaling axis make a clear understanding of its specific contributions necessary to understand how IL-17 mediates its effects.
IL-17RC and disease
Dysfunction of the IL-17 signaling axis has been implicated in a number of human diseases. As expected given its central role in regulating inflammation, IL-17 mediates many autoimmune diseases and protects against a variety of extracellular pathogens (reviewed in [3]). Most evidence to date suggests that IL-17RC contributes to human disease pathogenesis resulting from its function in the IL-17 signaling axis rather than via a specific pathogenic role.
Currently, evidence indicates that both the IL-17 signaling axis and IL-17RC, specifically, may play an important role in the development of certain cancers, although the detailed mechanisms remain uncharacterized. The initial discovery of IL-17RC was based on its high levels of expression in human prostate cancer cells [25, 39, 51]. Specific overexpression of IL-17RC protects prostate cancer cell lines from TNFα-induced apoptosis. This protection is not mediated through any of the canonical survival pathways such as via Bcl-2 and Bcl-XL and is apparently not dependent on any known IL-17-family ligands. Thus, IL-17RC may interact with novel signaling intermediates and ligands in the prostate cancer setting, although none has been identified [25].
Furthermore, in the murine B16 melanoma tumor model, IL-17A mediates a protumor function that involves IL-6 induction to activate Stat3 in both tumor cells as well as the tumor microenvironment [52]. IL-6 activation of Stat3 in tumor cells results in increases in anti-apoptotic, pro-proliferative, and pro-angiogenic genes, as well as suppression of certain proinflammatory genes [53]. In further support of the contribution of IL-17 signaling and IL-17RC to tumor regulation, ectopic overexpression of IL-17A by cervical carcinoma, fibrosarcoma, and lung cancer cell lines results in accelerated growth in vivo [54–56]. In humans, IL-17A-positive ovarian and lung cancer specimens displayed higher levels of angiogenesis compared to those devoid of IL-17A [54, 57].
The specific function of IL-17A and signaling via the IL-17R complex in the cancer setting, however, remains controversial [58–61]. Ectopic overexpression of IL-17A in hematopoietic tumor and sarcoma cells leads to enhanced antitumor immunity in immunocompetent mice [62, 63]. Growth enhancement of metastatic colon cancer cells in IL-17A-deficient mice with an accompanied reduction in interferon (IFN)γ-producing natural killer and tumor-specific T cells supports a protective function for IL-17A [64]. Moreover, adoptive transfer of Th17 or IL-17-positive CD8+ T cells resulted in tumor regression in the B16 melanoma model, albeit in an IFNγ-dependent and IL-17-independent manner [65, 66]. These differences likely result from the different tumor models originating from tumors of different tissues. It is conceivable that the differences in tissue distribution of IL-17RC and IL-17RA may account for some of the differences in biologic activity of IL-17, but this issue has not been defined.
IL-17RC also contributes to autoimmune disease pathogenesis. The role of Th17 cells in autoimmunity is beyond the scope of this review, but a few reports highlight the contributions of IL-17RC to this spectrum of diseases. The IL-17 signaling axis has been implicated as pathogenic in the development of rheumatoid arthritis (RA), which is characterized by the chronic inflammation of synovial tissues in multiple joints associated with bone and cartilage damage. IL-17A knockout mice develop significantly less severe arthritis, and treatment with IL-17A neutralizing antibodies or soluble receptors alleviates joint inflammation [67–69]. Certain animal models that develop spontaneous arthritis including the IL-1Ra-deficient mice and SKG mice also require IL-17A expression for disease progression [70, 71]. Moreover, human RA patients have high levels of IL-17A, IL-17F, IL-17RA, and IL-17RC in sera and inflamed synovium [72–74]. Higher levels of synovial IL-17A are predictive of more severe joint damage in RA, supporting a causal role [75]. Furthermore, based on RNAi blocking experiments, both IL-17RA and IL-17RC are required for the pro-inflammatory factors secreted by RA synoviocytes [76]. These studies suggest that to effectively treat RA, the effects of IL-17RC signaling should be targeted.
Impaired IL-17 signaling also drives the pathogenesis and disease progression of psoriasis, a chronic inflammatory disease of the skin characterized by epidermal hyperplasia, dermal angiogenesis, and leukocytic infiltration. IL-17A and IL-17F are overexpressed in the serum and lesions of psoriatic patients [77–79]. Surprisingly, gene transcript analyses of psoriatic lesions revealed an impairment of IL-17RC mRNA expression [80]. Perhaps this defect in IL-17RC expression leads to a compensatory effect, which could result in overactive Th17 cells and an inflammatory program. Accordingly, clinical targeting of Th17 cells by antibodies that neutralize the IL-12/IL-23 p40 subunit results in a significant improvement in disease pathology in psoriatic patients, associated with a decrease in the mRNA of the p19 subunit of IL-23 [81, 82]. Thus, neutralizing a cytokine required for Th17 cell survival in the psoriatic setting results in clinical benefit, underscoring the role of aberrant IL-17 signaling in disease.
Although the specific contribution of IL-17 to the development of inflammatory bowel disease (IBD) remains unclear, there is good evidence that IL-17 signaling affects the development and progression of this disorder. In mice, T cells deficient in IL-17A or IL-17RA mediate a more severe form of colitis, indicating a protective role for IL-17A in an adoptive transfer model of IBD [50]. Furthermore, IL-17A also serves a protective function in a chemically induced model of colitis [49]. IL-17F, however, was found to be pathogenic in this model of colitis, inducing the secretion of pro-inflammatory chemokines that exacerbated disease [48]. Additionally, IL-17F expression correlates with IBD development. Specific to the IBD spectrum, Crohn’s disease patients have significantly higher intestinal IL-17F levels than ulcerative colitis patients [83]. A report investigating the in vitro effects of IL-17A noted that it inhibits intestinal epithelial cell proliferation, an important mechanism of repair necessary to maintain epithelial integrity in a disease setting that destroys intestinal epithelium [84]. The functional differences between IL-17A and IL-17F in IBD may result from tissue-specific distribution of IL-17RC and IL-17RA because of the high affinity of IL-17F for IL-17RC. The relative levels of the receptors and the existence of soluble variants of IL-17RC may drive the nuanced differences the cytokines play in the different models of IBD.
The IL-17 signaling axis may also contribute to cardiac pathology and be amenable to therapeutic targeting. IL-17A correlates with autoimmune myocarditis, an inflammatory condition thought to result from immune targeting of cardiac myosin following myocardial infection [85]. Moreover, neutralization of IL-17A leads to alleviation of disease in an experimental myocarditis model [86, 87]. IL-17A stimulation of cardiac fibroblasts results in the production of a number of metalloproteases, suggesting that it may be partially responsible for tissue destruction and allowing access to inflammatory cells [88]. Furthermore, high glucose treatment of cardiac fibroblasts results in higher levels of IL-17A and IL-17RA expression but fails to modulate IL-17RC expression [89]. Thus, in hyperglycemic conditions, IL-17A and IL-17RA may potentiate myocardial inflammation, injury, and remodeling.
Given the role of IL-17RC as a receptor for IL-17F, evidence linking IL-17F to airway inflammation suggests that IL-17RC may be instrumental in this setting. Transgenic lung-specific IL-17F expression in mice caused lymphocytic and macrophage infiltration and mucus hyperplasia [48]. Furthermore, neutrophil recruitment associated with allergen-induced airway inflammation by Aspergillus protease extract is reduced in IL-17F-deficient but not in IL-17A-deficient mice [90]. However, this reduction is also observed in IL-17RA-deficient mice, suggesting that IL-17F potentiates allergen responses in an IL-17RA-dependent and probably IL-17RC-dependent manner. In an ovalbumin–alum-induced asthma model, IL-17F restricts the pathogenic role of Th2 cells, whereas IL-17A and IL-17RA promote Th2 responses in the lung [48, 91, 92]. While the specific role of IL-17RC has not been investigated in this setting, it is highly likely that IL-17RC also mediates these differences. In humans, a polymorphism in IL-17F leading to a missense mutation (His161Arg) is linked with protection against asthma development in a Japanese population [93]. Again, it is tempting to speculate that IL-17RA and IL-17RC expression levels skew the differences in cytokine function and biologic activity, but the role of IL-17RC is unknown. Additionally, IL-17 clearly plays an integral role in host defense against extracellular pathogens, specifically gram negative bacteria and fungi at mucosal surfaces [9, 94]. Further studies are needed to determine if the effects of IL-17RC in this setting extend from its role in mediating the IL-17 signaling axis or from an IL-17RC-specific function.
Recent studies have also shown that IL-17 affects bone formation and remodeling. IL-17 clearly plays a pathogenic, bone destructive role in arthritis, in part by inducing the expression of RANKL on osteoblasts and other mesenchymal cells [95–97]. Accordingly, IL-17A, IL-17RA, and IL-17RC have been found to localize to the site of bone fracture and the epiphyseal growth plate [98]. Counter to this, signaling via IL-17RA protects against periodontal bone loss in a mouse oral Porphyromonas gingivalis infection model, through a neutrophil recruitment mechanism [99]. Recently, using a murine model of ovariectomy-induced osteoporosis, IL-17RA signaling was shown to protect from systemic bone loss due to estrogen deficiency [100]. Thus, IL-17 signaling may directly affect bone remodeling and organization in the sterile settings of arthritis and fracture healing, while indirectly protecting from bone loss in other conditions such as infection or hormonal imbalances. The specific role of IL-17RC in these settings has not been defined.
Taken together, these studies illustrate the potential role of IL-17RC in disease pathogenesis and progression. IL-17RC may contribute directly to disease pathology or have indirect effects owing to its function as a receptor for IL-17F and as a subunit for the IL-17R complex. Either way, IL-17RC serves as an attractive therapeutic target in IL-17-dependent diseases.
Implications and conclusions
Although IL-17RC may act mainly through its function as an integral part of the IL-17R signaling complex, emerging data indicate that IL-17RC may play a more broad and nuanced role. The differences in genomic structure, splicing, and tissue distribution of IL-17RC compared to IL-17RA raise the prospect that IL-17RC may have distinct and/or complementary activities and thus provide a level of control and modulation of cytokine signaling. Although these findings are provocative, much more work is needed to dissect the molecular biology and biological activities of this system. In terms of therapeutic implications, the biology and function of IL-17RC in disease pathogenesis suggest that it may be exploited as a clinical target. Due to the relatively similar affinities of the cytokines IL-17A and IL-17F to hIL-17RC, soluble hIL-17RC molecules may prove to be effective in treating inflammatory diseases, including autoimmunity and certain cancers. No doubt the next few years will bring new insights into this intriguing member of the IL-17 receptor family.
References
Yu JJ, Gaffen SL (2008) Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 13:170–177
Ghilardi N, Ouyang W (2007) Targeting the development and effector functions of TH17 cells. Semin Immunol 19:383–393
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 cells. Annu Rev Immunol 27:485–518
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
Shen F, Ruddy MJ, Plamondon P, Gaffen SL (2005) Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol 77:388–399
Ouyang W, Kolls JK, Zheng Y (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454–467
Khader SA, Gaffen SL, Kolls JK (2009) Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2:403–411
McGeachy MJ, Cua DJ (2008) Th17 cell differentiation: the long and winding road. Immunity 28:445–453
O’Quinn DB, Palmer MT, Lee YK, Weaver CT (2008) Emergence of the Th17 pathway and its role in host defense. Adv Immunol 99:115–163
Aggarwal S, Gurney AL (2002) IL-17: a prototype member of an emerging family. J Leukoc Biol 71:1–8
Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL (2001) IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem 276:1660–1664
Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985–995
Moseley TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14:155–174
Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA (2007) An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 179:7791–7799
Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM (2007) Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem 282:13447–13455
Chang SH, Dong C (2007) A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res 17:435–440
Lubberts E (2008) IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 41:84–91
Garrett-Sinha LA, John S, Gaffen SL (2008) IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol 20:519–525
Tsang M, Friesel R, Kudoh T, Dawid IB (2002) Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol 4:165–169
Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA (2001) IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J 20:5332–5341
Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J (2006) Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 177:36–39
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567
Kramer JM, Yi L, Shen F, Maitra A, Jiao X, Jin T, Gaffen SL (2006) Evidence for ligand-independent multimerization of the IL-17 receptor. J Immunol 176:711–715
Kramer JM, Hanel W, Shen F, Isik N, Malone JP, Maitra A, Sigurdson W, Swart D, Tocker J, Jin T, Gaffen SL (2007) Cutting edge: identification of a pre-ligand assembly domain (PLAD) and ligand binding site in the IL-17 receptor. J Immunol 179:6379–6383
You Z, Shi XB, DuRaine G, Haudenschild D, Tepper CG, Lo SH, Gandour-Edwards R, de Vere White RW, Reddi AH (2006) Interleukin-17 receptor-like gene is a novel antiapoptotic gene highly expressed in androgen-independent prostate cancer. Cancer Res 66:175–183
Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK (1995) Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811–821
Shen F, Gaffen SL (2008) Structure–function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine 41:92–104
Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL (2004) Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem 279:2559–2567
Qian Y, Zhao Z, Jiang Z, Li X (2002) Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc Natl Acad Sci USA 99:9386–9391
Schwandner R, Yamaguchi K, Cao Z (2000) Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med 191:1233–1240
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256
Chang SH, Park H, Dong C (2006) Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281:35603–35607
Li X (2008) Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine 41:105–113
Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F (2003) The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci 28:226–229
Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL (2007) Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci USA 104:7506–7511
Ye H, Park YC, Kreishman M, Kieff E, Wu H (1999) The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol Cell 4:321–330
Park YC, Ye H, Hsia C, Segal D, Rich RL, Liou HC, Myszka DG, Wu H (2000) A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell 101:777–787
Park YC, Burkitt V, Villa AR, Tong L, Wu H (1999) Structural basis for self-association and receptor recognition of human TRAF2. Nature 398:533–538
Haudenschild D, Moseley T, Rose L, Reddi AH (2002) Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. J Biol Chem 277:4309–4316
Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y (2009) Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108–119
Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD (2007) Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 179:5462–5473
Iwakura Y, Nakae S, Saijo S, Ishigame H (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226:57–79
Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL (2008) Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol 181:4299–4310
Carter DB, Deibel MR Jr, Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN et al (1990) Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature 344:633–638
Rose-John S, Scheller J, Elson G, Jones SA (2006) Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol 80:227–236
Jones SA, Rose-John S (2002) The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta 1592:251–263
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289
Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C (2008) Regulation of inflammatory responses by IL-17F. J Exp Med 205:1063–1075
Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y (2004) Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 110:55–62
O’Connor W Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA (2009) A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol 10:603–609
Haudenschild DR, Curtiss SB, Moseley TA, Reddi AH (2006) Generation of interleukin-17 receptor-like protein (IL-17RL) in prostate by alternative splicing of RNA. Prostate 66:1268–1274
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H (2009) IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 206:1457–1464
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51
Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H (2005) IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 175:6177–6189
Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT (2003) Interleukin-17 promotes angiogenesis and tumor growth. Blood 101:2620–2627
Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautes-Fridman C (1999) Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res 59:3698–3704
Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T (2001) Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun 282:735–738
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W (2009) Phenotype, distribution, generation, functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149
Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W (2007) Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 178:6730–6733
Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z (2009) IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res 69:2010–2017
Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM (2008) Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 68:3915–3923
Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E (2002) Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 99:2114–2121
Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K (2001) Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology 61:79–89
Kryczek I, Wei S, Szeliga W, Vatan L, Zou W (2009) Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114:357–359
Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP (2009) Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 114:596–599
Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP (2008) Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112:362–373
Bush KA, Farmer KM, Walker JS, Kirkham BW (2002) Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum 46:802–805
Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB (2004) Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 50:650–659
Nakae S, Nambu A, Sudo K, Iwakura Y (2003) Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171:6173–6177
Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S (2007) T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med 204:41–47
Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y (2003) IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA 100:5986–5990
Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W (2000) High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol 164:2832–2838
Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P (1999) Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42:963–970
Hwang SY, Kim HY (2005) Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells 19:180–184
Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ (2006) Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum 54:1122–1131
Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, Miossec V, Miossec P (2008) IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol 180:655–663
Miossec P (2009) IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect 11:625–630
Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal MR, Kastelein RA, McClanahan TK, Bowman EP (2006) IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 203:2577–2587
Arican O, Aral M, Sasmaz S, Ciragil P (2005) Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005:273–279
Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K (2009) Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 160:319–324
Toichi E, Torres G, McCormick TS, Chang T, Mascelli MA, Kauffman CL, Aria N, Gottlieb AB, Everitt DE, Frederick B, Pendley CE, Cooper KD (2006) An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol 177:4917–4926
Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M (2007) A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 356:580–592
Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S (2008) Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis 14:437–445
Schwartz S, Beaulieu JF, Ruemmele FM (2005) Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem Biophys Res Commun 337:505–509
Furukawa Y, Kobuke K, Matsumori A (2001) Role of cytokines in autoimmune myocarditis and cardiomyopathy. Autoimmunity 34:165–168
Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M (2006) Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol 36:2849–2856
Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U (2006) T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med 203:2009–2019
Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B (2007) IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 293:H3356–H3365
Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B (2008) Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol 294:H2078–H2087
Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB (2007) A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol 120:334–342
Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B (2006) Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 203:2715–2725
Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y (2002) Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387
Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, Kokubu F, Maeda Y, Fukui Y, Konno S, Huang SK, Nishimura M, Adachi M (2006) IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol 117:795–801
Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL (2009) Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH (2009) IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ 16:1332–1343
Kokubu T, Haudenschild DR, Moseley TA, Rose L, Reddi AH (2008) Immunolocalization of IL-17A, IL-17B, and their receptors in chondrocytes during fracture healing. J Histochem Cytochem 56:89–95
Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL (2007) An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109:3794–3802
Goswami J, Hernandez-Santos N, Zuniga LA, Gaffen SL (2009) A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol 39:2831–2839
Acknowledgments
This work was supported by the Medical Scientist Training Program at the University at Buffalo (SUNY) School of Medicine and Biomedical Sciences. SLG was supported by the NIH (AR054389), Amgen, and the Alliance for Lupus Research. We thank Dr. Brett N. Tomson for valuable comments and assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ho, A.W., Gaffen, S.L. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol 32, 33–42 (2010). https://doi.org/10.1007/s00281-009-0185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-009-0185-0