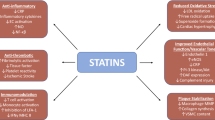
Abstract
Anti-inflammatory activities of statins in atherosclerosis have been well documented by both basic research and clinical studies. Statins have been introduced in the 1980s as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors to block cholesterol synthesis and lower cholesterol serum levels. In the last three decades, statins have been shown to possess several anti-inflammatory and antioxidant activities resulting in the beneficial reduction of atherosclerotic processes and cardiovascular risk in both humans and animal models. Inflammatory intracellular pathways involving kinase phosphorylation and protein prenylation are modulated by statins. The same intracellular mechanisms might also cause statin-induced myotoxicity. In the present review, we will update evidence on statin-mediated regulation of inflammatory pathways in atherogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are the leading cause of death and disability in the adult population of developed and developing countries [1–3]. In the majority of patients, clinical cardiovascular disease is the final step of the inflammatory state characterizing atheroprogression. Stable advanced atherosclerotic plaques mainly induce chronic arterial lumen stenosis and ischemia in peripheral tissues. This condition causes chronic organ remodeling and alters their functions. In the heart, chronic hypoxia can induce congestive heart failure (CHF). On the other hand, unstable plaques frequently progress to rupture with the consequent exposure to the blood lumen of intraplaque prothrombotic material. In that case, thrombus causes the sudden complete occlusion of the arterial lumen, and peripheral tissues are exposed to acute ischemia. If collateral arteries are not present and acute ischemia is prolonged, tissue necrosis can occur with dramatic consequences in the heart and brain. Several cardiovascular risk factors have been associated with CVD. More than 50 years ago, this concept was introduced by the Framingham Heart Study, with the identification of major coronary heart disease (CHD) risk factors, such as hypertension, hyperlipidemia, smoking, and diabetes [4]. However, although highly sensitive, these traditional factors showed a very low specificity [5–7]. Therefore, inflammatory soluble mediators, which have been shown to play a central role in all phases of atherosclerosis, have been investigated [8–10] with some preliminary encouraging results. In particular, the American Heart Association (AHA/CDC) has recently suggested that the high sensitivity dosage of the acute phase reactant C-reactive protein (CRP) might be useful when physicians are undecided about indications of a more intensive treatment for patients who are considered at intermediate cardiovascular risk [11, 12]. At present, the most promising therapeutic strategies to reduce cardiovascular diseases are represented by the selective blockade of both “classical” and new emerging factors promoting atherogenesis. Statins (3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors) should be considered as anti-atherosclerotic drugs capable of modulating different factors. Basic research and clinical studies have shown that statins lower LDL cholesterol levels and inhibit atherosclerotic inflammatory processes. Little is known on the possible statin-mediated molecular mechanisms to reduce cardiovascular risk. In the present review, we will focus on selective anti-inflammatory activities of members of statin family and the statin-mediated modulation of intracellular pathways in both immune and vascular cells, which play a crucial role in atherosclerosis.
Statins in cardiovascular diseases
The first identification of a fungal metabolite (compactin) blocking cholesterol synthesis has been performed in 1971 by Endo and coworkers [13]. In 1976, the “Compactin Development Project” composed by several experts started. Starting from 1978, a new statin (called lovastatin) has been discovered and developed. In 1980s, statins have been shown to lower LDL cholesterol levels in patients with hypercholesterolemia [14–18]. These first impressive results induced the US Food and Drug Administration to approve the commercial us of statins in 1986 [19]. After lovastatin, three generations of statins have been commercially introduced: pravastatin and fluvastatin (first generation), atorvastatin and simvastatin (second generation), and rosuvastatin and pitavastatin (third generation). Cerivastatin was also approved for the marketplace but was eventually discontinued for its myotoxic effects [13]. Statins have been tested in primary prevention for acute cardiovascular events in both high cardiovascular risk patients (diabetes, hypertension, and dyslipidemia) and subjects with low or no CVD risk [20–25]. For instance, aggressive treatment of diabetic subjects with statins has been shown to reduce coronary events and stroke [21–23]. This was also observed in diabetic patients with dyslipidemia [26]. The principal primary prevention study in hypertensive patients (Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA)) showed that atorvastatin reduces the relative risk of primary CHD [27]. On the other hand, The Intervention Trial Evaluating Rosuvastatin (JUPITER, a large multinational, long-term, double blind, and randomized clinical trial) showed that treatment with rosuvastatin significantly reduces the incidence of major cardiovascular events in healthy subjects without hyperlipemia but with elevated high-sensitivity CRP levels [28]. Statins have been also investigated in secondary prevention of cardiovascular diseases. Results have been reported in patients with stable coronary artery disease, acute coronary syndrome, stroke, or transient ischemic attack [29–36]. The Scandinavian Simvastatin Survival Study (4S), performed in patients with angina pectoris or previous myocardial infarction and high-serum cholesterol levels, demonstrated that long-term treatment with simvastatin improves survival and decreases cardiovascular events [37]. The Heart Protection Study (HPS; a primary and secondary prevention study) showed that simvastatin significantly reduced cardiovascular mortality and vascular acute events in 20,000 patients with coronary or peripheral vascular disease without hyperlipidemia [38]. The Greek Atorvastatin and Coronary Heart Disease Evaluation, Treating to New Targets (TNT), Aggressive Lipid-Lowering Initiation Abates New Cardiac Events, and Incremental Decrease in Endpoints Through Aggressive Lipid Lowering (IDEAL) studies confirmed a significant decrease of acute cardiovascular events in patients with stable CHD [39, 40]. The Pravastatin or Atorvastatin Evaluation and Infection Therapy, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL), and IDEAL-Acute Coronary Syndromes studies demonstrated the benefits of high-dose-dosage atorvastatin therapy in patients with acute coronary syndromes [40]. In patients with heart failure, the efficacy of statins is still a matter of dispute [41, 42]. In the GISSI-HF trial, rosuvastatin did not reduce death or admission to hospital for cardiovascular reasons in patients with chronic heart failure [42]. The CORONA study partially confirmed these results showing only a reduction of the number of cardiovascular hospitalizations [43]. Statins also improved survival and cardiovascular events in patients after heart transplantation or percutaneous coronary interventions [41] promoting coronary collateral circulation, a modest antihypertensive effect and improving glucose metabolism and insulin sensitivity [44–46]. Although few controversies remain, these clinical studies indicate that: (1) statins should be considered as very promising anti-atherosclerotic drugs (also independently on lowering cholesterol) for secondary prevention in all patients and for primary prevention in high risk individuals (Fig. 1). (2) In persons without traditional cardiovascular risk factors, but with elevated hs-CRP levels, the use of statins might be indicated in the near future. (3) The marked cardiovascular risk reduction might be also related to the direct anti-inflammatory and pleiotropic properties of statins [47–59]. The National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III recommended the use of statins for the secondary prevention of cardiovascular diseases as the first-line choice drug for lowering LDL-cholesterol in the high-risk patients [60]. This group includes individuals with established coronary heart disease and diabetes mellitus or with a Framingham 10-year CHD risk greater than 20%. For primary prevention, the NCEP ATP III recommended to treat individuals with metabolic syndrome and diabetic patients with moderate cardiovascular risk (Framingham 10-year CHD risk of 10–20%) [61]. Treatment with any of the statin family has been associated with transaminase elevations in comparison to placebo [62]. Despite the low frequency (1–2%) of raised asparatate or alanine aminotransferase, these adverse effects are completely reversible and almost never progress to hepatitis or liver failure [63]. A very low risk of neuropathy and gastrointestinal discomfort has been also reported. However, the main risk of statin treatment derives from myotoxicity which ranges from myalgias, creatine kinase elevation to the more serious rhabdomyolysis. This adverse effect has been reported in 1–7% of patients under statin therapy [64]. Itagaki and coworkers recently suggested that myotoxicity could be induced by the same mechanisms governing statin beneficial anti-inflammatory activities. In particular, RhoA dysfunction due to loss of lipid modification with the mevalonate product geranylgeranylpyrophosphate (GGPP) has been observed in statin-induced skeletal muscle toxicity [65]. Further investigations are needed to better clarify the role of intracellular signaling pathways in myotoxicity. Despite these few adverse effects, statins are generally well tolerated. Both second and third generations of statins have been shown to reduce LDL cholesterol more effectively than first generation without increasing toxicity [66]. In the following, we will discuss on the selective immunomodulatory properties of different statins (Table 1) [67].
Statin treatment reduces the risk of acute cardiovascular events. Several studies support that statins inhibit atherosclerotic plaque progression and the risk of rupture (primary prevention). These beneficial effects are mediated by statin-induced cholesterol reduction and statin-mediated “pleiotropic” activities. In secondary prevention, the use of statins has been suggested by promising results. However, the prompt restoration of antegrade flow in the infarct-related coronary artery, whether accomplished pharmacologically or mechanically, remain the mean therapy for improving both left ventricular systolic function and survival during the subsequent hours after the acute myocardial infarction. Further investigations are needed to investigate the possible use of statins to prevent post-ischemic diseases (such as CHF)
Lovastatin
Together with pravastatin and fluvastatin, lovastatin is included in the first generation of statins. Lovastatin represents the drug with the lowest potency and is necessary to use high doses to observe a significant reduction of LDL cholesterol levels [68]. However, lovastatin (together with simvastatin and pravastatin) is less expensive, and it is available in generic formulation. Clinical studies have also confirmed the efficacy of lovastatin in both primary and secondary prevention of cardiovascular diseases. The AFCAPS/TexCAPS study showed that daily treatment with 20–40 mg lovastatin-reduced incidence of acute major cardiac events in comparison with placebo. The study was performed in a middle-aged or elderly population (n = 6,605) without symptomatic cardiovascular disease [69]. The beneficial effects of lovastatin were confirmed by the ACAPS study that enrolled 919 subjects asymptomatic for clinical cardiovascular disease but with evidence of early carotid atherosclerosis [70]. On the other hand, secondary prevention studies showed that 2–2.5-year therapy with lovastatin-reduced progression of atherosclerosis in dyslipidemic patients with CHD [71–73]. Lovastatin has been also shown to induce in vitro anti-inflammatory activities in different cell types at lower doses. Lovastatin is a potent immunomodulatory and neuroprotective drug. It strongly reduces the migration of monocytes and lymphocytes across in vitro-cultured human blood–brain barrier endothelial cells [74]. Lovastatin also reduces apoptosis and downregulates CD40 expression in TNF-alpha-treated cerebral vascular endothelial cells and in IFN-gamma-treated microglial cells [75, 76]. Lovastatin-induced reduction of transendothelial T cell migration is dependent on the inhibition of rho pathway [77]. Lovastatin directly modulates immune cell functions by both disrupting or upregulating chemokine and chemokine receptor expression [78, 79]. Furthermore, lovastatin inhibits maturation and functional changes of bone marrow-derived dendritic cells [80]. Lovastatin also increases macrophage apoptosis involving the Rac1/Cdc42/JNK pathways [81]. Taken together, these studies support lovastatin as an immunomodulatory therapeutic agent in atherosclerosis and its cerebral complications.
Pravastatin
Pravastatin is the most rigorous tested drug in the “first generation” of statins. Two important primary prevention studies (WOSCOPS and PROSPER) indicated that pravastatin treatement (40 mg/daily) reduced LDL-cholesterol levels, cardiac mortality, and coronary events in comparison with placebo [82, 83]. ALLHAT-LLT study (enrolling 10,355 patients) did not confirm pravastatin-induced reduction in coronary events or mortality [84]. These different results in primary prevention were probably due to the lower reduction of LDL cholesterol levels (−28%) in ALLHAT-LLT study. Furthermore, a strong limitation was represented by the populations, which was exclusively enrolled in developed west countries. To investigate the efficacy of pravastatin in primary cardiovascular prevention in another population, the MEGA study was planned in Japan. Treatment with a low dose (10–20 mg) of pravastatin reduced the risk of coronary heart disease in Japan similarly to that observed in Europe and the USA in the presence of higher doses [85]. Differently from fluvastatin or simvastatin (that are “lipophilic”), pravastatin is a “hydrophilic” compound as the new rosuvastatin and almost not metabolized by the cytochrome P450 complex [86]. Probably for this property, treatment with pravastatin induced different effects in vascular cells. Wiesbauer and coworkers showed that among six different statins, only pravastatin does not decrease PAI-I production in human endothelial cells and smooth muscle cells [87]. Furthermore, pravastatin increases E-selectin and vascular cell adhesion molecule (VCAM)-1-induced expression on vascular endothelial cells stimulated with TNF-alpha or LPS [88]. This study also showed that treatments with simvastatin or fluvastatin increase adhesion molecule expression in TNF-alpha-stimulated endothelial cells [88]. Therefore, statins might increase TNF-alpha-mediated proinflammatory activities in endothelial cells. Conversely, pravastatin has been shown to protect mouse cerebral endothelial cells against ceramide-mediated cytotoxicity through vascular endothelial growth factor upregulation and the activation of hypoxia-inducible factor-1 [89]. Furthermore, pravastatin inhibits the production of monocyte chemoattractant protein 1 (MCP-1), interleukin (IL)-6, and IL-8 in irradiated microvascular endothelial cells [90]. These beneficial effects were persistent up to 14 days after irradiation, suggesting that pravastatin could be considered a very promising agent against endothelial dysfunction in patients treated with radiation therapy. These controversial data suggest a still unclear immunomodulatory role of pravastatin on vascular cells. Recent evidence also showed a possible role of pravastatin in vascular endothelial cell protection from oxidative stress. In a rat model of myocardial infarction, Abe and coworkers showed that pravastatin prevented cardiac dysfunction. The suggested mechanism for pravastatin-induced beneficial effects was represented by the inhibition of H2O2-mediated caspase-3 activation and endothelial nitric oxide synthase (eNOS) reduction that was observed in cultured vascular endothelial cells [91]. More recently, Suzuki and coauthors showed that pravastatin also improves dysfunctional hibernating myocardium in a swine model of chronic left anterior descending artery (LAD stenosis) [92]. These investigations clearly indicate that improvement after pravastatin administration was exclusively related to mobilization of CD133(+)/cKit(+) bone marrow progenitor cells, without involving endothelial cell function. Controversial immunomodulatory properties of pravastatin have been also shown in immune cells [93]. Pravastatin increases inflammation by activating human peripheral blood mononuclear cells to produce IL-18, TNF-alpha, and IFN-gamma [94]. Furthermore, it increases the phagocytic index of mouse peritoneal macrophages [95]. Conversely, pravastatin induces important immunosuppressive effects by prolonging lung graft survival and inhibiting chronic rejection in renal allograft in rats [96, 97]. Pravastatin treatment also inhibits circulating dendritic cell activation in patients with coronary artery disease [98].
Fluvastatin
Fluvastatin is a lipophilic statin. Differently from other statins that are metabolized by the cytochrome P450 3A4 complex (CYP3A4), fluvastatin is metabolized by another P450 complex [99]. This aspect reduces the risk of pharmacological interactions between fluvastatin and other drugs. Furthermore, it suggests the possible use of fluvastatin in patients, which are intolerant to second and third generation of statins. The ALERT study investigated the possible reduction of cardiovascular risk in patients who received renal transplant. Fluvastatin treatment reduced cardiac deaths and nonfatal MI but not coronary intervention procedures or mortality in these patients [100]. In two other secondary prevention studies (BCAPS and HYRIM) respectively conducted in patients who had carotid plaque without symptoms of carotid artery disease [101] and hypertensive men [102], fluvastatin-reduced acute cardiovascular events. On the other hand, the Lescol Intervention Prevention Study showed that fluvastatin treatment significantly reduces the risk of adverse cardiac events in patients with average cholesterol levels undergoing their first successful percutaneous coronary intervention [103]. Fluvastatin has been shown to induce cardioprotective effects in both acute and chronic treatments. In a rat model of acute myocardial infarction and reperfusion, the administration of fluvastatin 20 min before the onset of ischemia significantly attenuated the decline of myocardial blood flow, thus, reducing myocardial infarction size [104]. These promising data are in contrast with in vitro studies showing that fluvastatin induces cardiac myocyte and vascular endothelial cell apoptosis [105, 106]. Furthermore, it increases the expression of adhesion molecules, MCP-1, and tissue factor in human umbilical vein endothelial cells stimulated by antiphospholipid antibodies [107]. It is possible that fluvastatin exerts its activities in endothelial cells through its uptake into these cells via nonspecific simple diffusion [108]. These apparent negative effects might be counterbalanced by other protective activities induced by fluvastatin in vascular cells. In fact, fluvastatin inhibits matrix metalloproteinase-1 expression and oxidative damage in vascular endothelial cells, thus, improving endothelial dysfunction [109–112]. Fluvastatin also increases prostacyclin production and reduces endothelin-1 secretion in human umbilical vein endothelial cells (HUVEC) contributing to vasodilation and, thus, preventing cardiovascular diseases [113]. At very high concentration (10 μM), fluvastatin also reduces CRP-induced TNF-alpha secretion in HUVEC [114]. Despite the important limitation represented by the high dose used, this study supports a direct activity of fluvastatin in the inhibition of CRP-mediated proinflammatory effects. Fluvastatin has been shown to reduce inflammatory cells activation and functions. Mononuclear leukocytes represent the most investigated cell population [115–117]. The anti-inflammatory properties of fluvastatin have been observed in patients with chronic heart failure or allergic asthma [118, 119]. These studies suggest the potential therapeutic use of fluvastatin not only in atherosclerosis but also in other inflammatory diseases.
Simvastatin
Together with atorvastatin, simvastatin has been actively investigated [120], and it has been shown to induce several in vitro and in vivo beneficial effects to reduce atherosclerotic inflammatory process. Clinical trials in primary and secondary prevention of cardiovascular risk demonstrated the beneficial effects of simvastatin. In primary prevention, the HPS provided direct evidence that cholesterol-lowering therapy-reduced cardiac mortality and coronary events mainly in diabetic subjects without a manifest coronary disease [121]. In secondary prevention, the 4S showed that treatment with simvastatin strongly reduced cardiac mortality and coronary events in comparison to placebo [37]. Simvastatin reduces endothelium dysfunction through a direct benefit on endothelial cells and their precursors [122]. High concentrations of simvastatin (25 μM) reduced TNF-alpha-mediated apoptosis of endothelial progenitor cells (EPC) [123]. Furthermore, the treatment with 80 mg/day simvastatin for 5 days increases in vivo EPC mobilization [124]. Then, simvastatin also promotes endothelial differentiation of bone marrow stromal cells through the Notch signaling pathway [125]. On the other hand, simvastatin might prevent endothelial dysfunction through a direct protective activity on endothelial cells. Simvastatin inhibits CRP-induced CD32, VCAM-1 expression as well as monocyte adhesion to human umbilical vein endothelial cells [126]. Simvastatin treatment also inhibits TNF-alpha-induced adhesion molecule (selectins) expression on HUVEC [127]. Furthermore, simvastatin protected the vascular endothelium against damages induced by LDL or ox-LDL in rats or a cultured cell line (ECV304 cells) [128]. On the other hand, emerging evidence shows that simvastatin also increases endothelial cell apoptosis suggesting that the clinical benefit of simvastatin in endothelial dysfunction could be due to an unbalance between positive (endothelial cell and endothelial progenitor protection) and negative (endothelial cell apoptosis) activities [129–131]. Simvastatin has been also shown to modulate eNOS in humans and animal models, thus, contributing to reduce endothelial dysfunction [132, 133]. The inflammatory functions of other vascular and immune cell types involved in atherosclerotic processes have been modulated by simvastatin. Simvastatin induces the cytoprotective molecule heme oxygenase-1 in mouse smooth muscle cells in vivo and in vitro [134]. Treatment with 1 μM simvastatin significantly downregulates chemokine and chemokine receptor expression in human macrophages [135]. These morphological changes result in a reduced locomotory response towards CCL2, as shown in vivo by Han and coworkers [136]. More recently, we showed that simvastatin at 1 μM reduces CRP-induced chemokine secretion, ICAM-1 upregulation, and chemotaxis in human primary monocytes cultured in adherence to polystyrene [137]. In CD14+ monocytes, simvastatin reduces toll-like receptor 4 expression suggesting that this drug might directly modulate innate immunity [138]. Neutrophil migration, adherence, and membrane integrity have been also modulated by treatment with simvastatin [139, 140]. Simvastatin also interferes with inflammatory activities mediated by humoral and cell-mediated immunity in vivo and in vitro [141]. Simvastatin inhibits both human B- and Th1-lymphocyte activation [142–144]. On the other hand, simvastatin promotes Th2-type responses through the direct modulation of dendritic cell function [145]. Accordingly with these data, simvastatin treatment in vivo inhibits lymphocyte and NK cell functions and downregulated angiotensin II type 1 receptor on circulating T lymphocytes and monocytes [146, 147].
Atorvastatin
Atorvastatin is the first statin approved to reduce the risk of hospitalization in heart failure patients [148]. Large clinical trials have been performed to assess primary and secondary prevention of cardiovascular diseases. In primary prevention, the ASCOT-LLA and the Collaborative Atorvastatin Diabetes Study showed a significant reduction in cardiovascular events with 10 mg/day atorvastatin in comparison to placebo [27, 149]. In secondary prevention, the majority of clinical studies performed investigated high dose treatments versus low dose. In the TNT and in the IDEAL studies, intensive atorvastatin treatment (80 mg/day) reduced any coronary events in comparison to lower statin doses (respectively 10 mg/day atrovastatin and 20 mg/day simvastatin) in patients with stable coronary artery disease or a history of acute myocardial infarction. In 2001, the MIRACL study further confirmed the need of high-dose atorvastatin therapy to prevent recurrent coronary events in patients with recent acute coronary syndrome. However, in all these studies, no reduction in mortality was observed [30–32, 141–149]. The Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 is the only study showing a reduction in mortality in the presence of intensive atorvastatin treatment in secondary prevention of acute cardiovascular events [150]. Chronic treatments with atorvastatin have been shown to induce direct beneficial effects in cardiomyocytes by protecting from hypertrophic cardiomyopathy, cardiac fibrosis, and remodeling, catecholamine deleterious effects in several animal models [151–154]. Several studies investigating the possible effect of atorvastatin in inflammatory diseases have been performed with some controversial results [155–157]. For instance, atorvastatin treatment failed to reduce inflammatory processes in a mouse model systemic lupus erythematosus [157]. Atorvastatin has been also shown to directly induce neuroprotection. It reduced neurological deficit by increasing synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury [158]. In the same rat model, atorvastatin also reduced intravascular thrombosis and increased cerebral microvascular integrity [159]. Furthermore, atorvastatin inhibited perihematomal cell death in adult rats after experimental intracerebral hemorrhage [160]. As other statins, atorvastatin directly antagonized inflammatory processes in human brain endothelial cells [161]. The direct neuro and endothelial protective activities of atorvastatin have been shown to reduce stroke consequences in stroke-prone spontaneously hypertensive rats and in a rat model of embolic stroke [162, 163]. These studies strongly suggest that atorvastatin could be a very promising drug with potent anti-inflammatory activities in different diseases. The possible use of atorvastatin to inhibit atherosclerosis represents the best investigated field. Atorvastatin reduced in vitro and in vivo endothelial dysfunction and inflammatory processes in atherogenesis. As previously indicated for simvastatin, atorvastatin protects endothelium through the increase of endothelial cell progenitor proliferation and mobilization and the induction of vessel wall stabilizing mediators and growth factors in endothelial cells [111, 164–166]. Atorvastatin has been shown to inhibit cytokine-inducible nitric oxide synthase, CD40, CD40L, chemokine, and thrombospondin-1 expression in endothelial cells [167–172]. Atorvastatin also regulates immune cell functions and the release of inflammatory mediators such as CRP. We have recently shown that atorvastatin inhibit CRP production on IL6-treated human hepatocytes [173]. Furthermore, this drug reduces CRP or granulocyte macrophage–colony stimulating factor-mediated proinflammatory activities in human monocytes [137, 174]. Atorvastatin also interferes with T lymphocyte, B lymphocyte, or dendritic cell activation and differentiation [175–179]. Atorvastatin-mediated anti-inflammatory effects have been observed also in clinical studies, investigating inflammatory cardiovascular risk markers in acute coronary syndromes, and coronary artery disease [180, 181]. Further investigations are needed to better assess the role of atorvastatin treatment in the reduction of inflammatory cardiovascular risk factors. Taken together, clinical and basic research studies suggest that high-dose atorvastatin treatments rather than low-dose could be considered as a promising approach to reduce cardiovascular disease.
Rosuvastatin
Rosuvastatin is a synthetic HMG-CoA reductase inhibitor noncompetitive with the cosubstrate nicotinamide-adnine dinucleotide phosphate [182]. Compared to other statins, rosuvastatin is the most effective to reduce total and LDL-cholesterol levels for mg/dose equivalent. As atorvastatin, rosuvastatin has been shown to additionally bind the enzyme complex not only at the HMG-CoA reductase site [183]. As pravastatin, it is significantly less lipophilic than other statins. Differently from other statins, rosuvastatin is metabolized mainly through CYP2C9 [183]. These pharmacologic and pharmacokinetic aspects support this drug as a very promising candidate to reduce myotoxicity and interaction with other drugs, and improve anti-inflammatory properties. Several clinical studies included in the global GALAXY program have been performed to assess rosuvastatin’s clinical efficacy in atherogenic lipid profile, changes in atheroma volume, and cardiovascular morbidity and mortality in different subpopulations [184]. As described above, the recent JUPITER study is the first primary prevention study demostrating a benefit of statin therapy in individuals with elevated high-sensitivity C-reactive protein levels, but without hyperlipidemia [28]. In secondary prevention, rosuvastatin did not reduce mortality in patients with systolic heart failure and CAD but only the number of cardiovascular hospitalizations. Conversely, the ASTEROID trial demonstrated decrease of percent diameter stenosis and amelioration of minimum lumen diameter in coronary disease patients [185]. Although atherosclerotic lesion regression is not directly related to the reduction of future cardiovascular outcomes, the ASTEROID study strongly supports the possible use of rosuvastatin in secondary prevention. High concentrations of rosuvastatin (100 μM) suppressed monocytic cell adhesion and ICAM-1, MCP-1 IL-8, IL-6, and COX-2 expression on TNF-alpha-stimulated HUVEC [186]. Furthermore, although mechanisms have not been identified yet, rosuvastatin treatment protected mice from ischemic stroke [187] and rats from myocardial reperfusion injury [188]. Rosuvastatin also exerts favorable anti-atherosclerotic effects in several animal models [189–192]. These studies suggest that rosuvastatin-mediated benefits are dependent of its antioxidant and anti-inflammatory activity on endothelial cells. A recent study (performed in a small number of patients (n = 48)) further confirmed the endothelial protective properties of rosuvastatin showing that short-term treatment increases endothelial cell progenitor mobilization [193].
The effects of statins on inflammatory intracellular signaling pathways
Statins have been introduced as HMG-CoA reductase inhibitors to lower LDL-cholesterol synthesis and serum levels (Fig. 2). However, emerging evidence has shown that the “classical” cholesterol pathway could also modulate intracellular signaling pathways involving protein kinases in different cell types. Thus, statins could directly influence vascular and immune cells, hepatocyte, and adipocyte inflammatory functions through the activation of certain intracellular mediators or the inhibition of protein kinase phosphorylation of intracellular second messenger cascades (Fig. 3).
Anti-inflammatory activities of stains depend on both “classical” and “not classical” HMGCoA-Mevalonate pathways. Simplified “classical” and “not classical” HMGCoA-Mevalonate pathways. Statin anti-inflammatory activities depend on the inhibition of both “classical” (HMGCoA–Mevalonate–FPP–cholesterol) and “not classical” (HMGCoA–Mevalonate–FPP–kinase) pathways. Other studies have shown that GGPP may be also involved in the intracellular signaling proteins regulating kinase activation
“Activatory” and “inhibitory” intracellular signaling pathways regulate statin-induced pleiotropic activities. Statins diminish proinflammatory effects and promote anti-inflammatory activities through the direct inhibition/activation of chemokine-, cytokine-, and acute phase reactant-induced intracellular signaling pathways in several cell types (such as leukocytes, vascular cells, adipocytes, and hepatocytes). In particular, the inhibition of ERK 1/2, rho, JAK/STAT3, or mevalonate pathways is crucial to reduce inflammation. On the other hand, statins directly activates AMPK and PI3K/Akt/NFkB pathways, thus, increasing cell survival, endothelial, and neuronal protection
Statin-mediated activation of intracellular pathways
Recently, statins have been shown to directly activate anti-inflammatory intracellular pathways. AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase involved in the regulation of cellular energy metabolism. Emerging evidence showed that AMPK might be crucial in vascular protection [194]. Statins directly activates AMPK in different cell types [194, 195]. In particular, statins have been shown to rapidly activate this protein kinase in vitro (cultured HUVEC) and in vivo (mouse aorta and myocardium). Such phosphorylation resulted in eNOS activation that could explain the direct beneficial effects of statins in cardiovascular diseases [196]. Interestingly, statin-mediated AMPK activation was dependent on the upstream activation of PKC-zeta as shown in endothelial cells and in vivo in mice [197]. AMPK-induced eNOS activation also increased endothelial cell progenitor differentiation in endothelial cells indicating a possible protective mechanism on endothelium mediated by statins [198].
On the other hand, the activation of phosphoinositide 3 kinase (PI3K)/Akt (protein kinase B) represents a critical step in inflammatory cell survival, locomotion in atherosclerosis, and cardiovascular disease [199]. Although, few studies (performed with lovastatin) suggested a possible inhibition of PI3K-Akt in endothelial cells [200], there is a general consensus that statins induce cardiovascular protection through the activation of PI3K/Akt pathway. Statin-mediated neuroprotection in animal models of traumatic brain injury and embolic stroke is dependent on PI3K/Akt pathway activation [163, 201, 202]. This pathway involves also the activation of NF-kB that suppresses caspase-3 and apoptosis cell death [203]. Neuronal nitric oxide synthase upregulation might represent the final effector of this protective pathway [204]. Statin-mediated myocardial protection has been also shown as dependent on PI3K/Akt activation pathway in both in vitro and in vivo models [205–207]. Atherogenesis is also regulated by PI3K/Akt pathway. Statins induce their activation on vascular and immune cells, thus, reducing atherosclerotic lesions in ApoE knockout mice [208] and endothelial dysfunction in type 2 diabetic mice [209]. Controversial results on statin-mediated MAPK phosphorylation have been showed. Statins induce anti-inflammatory activities through both activation and inhibition of these kinases. The statin-induced activation of extracellular signal-regulated kinase (ERK) 1/2 has been observed mainly in immune cells [210, 211]. On the other hand, statins increase macrophage apoptosis through JNK activation [81].
Statin-mediated inhibition of intracellular pathways
As largely discussed, statins have been approved as HMG-CoA reductase inhibitors. The HMG-CoA/mevalonate pathway is essential for cellular metabolism and the formation of precursors of bile acids, lipoproteins and hormones, and nonsterol isoprenoids. These molecules regulate cell growth and differentiation, gene expression, protein glycosylation, and cytoskeletal assembly [212]. Thus, by influencing cholesterol synthesis, statins not only reduce circulating LDL-cholesterol levels but also induce important cellular modifications which could interfere with immune and thrombotic response. Furthermore, statin reduce mevalonate-derived molecules, such as farnesylpyrophosphate (FPP) and GGPP which are essential players in cell signaling, endocytotic/exocytotic transport, and cytoskeleton dynamics [213]. Immune cell functions (including chemotaxis, chemokine secretion, and oxidative metabolism) are regulated by the “classical” mevalonate pathway. Endothelial and smooth muscle cells require this pathway for their proinflammatory functions [213]. Therefore, anti-inflammatory properties of stains might be due to their “classical” pharmacologic properties. Small GTP-binding proteins, which include in the Ras, Rho, Rab, and Ran superfamily, are directly modulated by the mevalonate pathway. These proteins act as molecular “on–off” switches regulating actin cytoskeleton changes in cell adhesion, migration and contraction [214]. They regulate several downstream intracellular messengers, such as protein kinase N, p21-activated protein kinase, and the rho-associated kinases, which control not only cellular locomotion but also cell growth and apoptosis in both immune and vascular cells [215]. Recently, it has been shown that the effects of statins on atherosclerotic processes in the vessel wall and in the brain are partially due to the inhibition of the Rho pathway [216, 217].
Conclusion
Due to their beneficial properties in cardiovascular diseases, statins are among the most widely prescribed medications in the world. Despite some differences between members of the statin family, all these drugs have been shown to reduce cardiovascular events in both primary and secondary prevention. The recent results of the JUPITER Study seem to indicate that statin treatment in CRP-elevated primary prevention markedly reduced cardiovascular events and total mortality. The safety of statins has been largely documented. Beside their well-known lipid lowering effects, statins also affect some immunomodulatory activities. These so called “pleiotropic” effects induce a decrease in pro-inflammatory and an increase in anti-inflammatory molecular pathways. Statin-mediated modulation of molecular intracellular mechanisms in both vascular and immune cells should be considered as a very promising approach to further clarify the statin-induced cardiovascular protection. Beside cardiovascular area, statin therapy is under investigation in other inflammatory diseases, such as rheumatoid arthritis and multiple sclerosis. Many hypotheses have been already proposed to explain statin anti-inflammatory activities. A strong work is waiting for researchers in the next years.
References
Yusuf S, Reddy S, Ounpuu S et al (2001) Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104:2746–2753
Yusuf S, Reddy S, Ounpuu S et al (2001) Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 104:2855–2864
American Heart Association (2002) 2002 Heart and Stroke Statistical Update. American Heart Association, Dallas
Wilson PW, D’Agostino RB, Levy D et al (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847
Khot UN, Khot MB, Bajzer CT et al (2003) Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 290:898–904
Root M, Cobb F (2004) Traditional risk factors for coronary heart disease (letter). JAMA 291:299
Greenland P, Knoll MD, Stamler J et al (2003) Major risk factors as antecedents of fatal and non fatal coronary heart disease events. JAMA 290:891–897
Carter AM (2005) Inflammation, thrombosis and acute coronary syndromes. Inflammation, thrombosis and acute coronary syndromes. Diab Vasc Dis Res 2:113–121
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6:508–519
Hansson GK, Libby P, Schöenbeck U et al (2002) Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 91:281–291
Pearson TA, Mensah GA, Alexander RW et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511
Libby P, Ridker PM (2004) Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 116(Suppl 6A):9S–16S
Endo A (2008) A gift from nature: the birth of the statins. Nat Med 14:1050–1052
Tobert JA, Bell GD, Birtwell J et al (1982) Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase, in healthy volunteers. J Clin Invest 69:913–919
Tobert JA, Hitzenberger G, Kukovetz WR et al (1982) Rapid and substantial lowering of human serum cholesterol by mevinolin (MK-803), an inhibitor of hydroxymethylglutaryl-coenzyme A reductase. Atherosclerosis 41:61–65
Hoeg JM, Brewer HB Jr (1987) 3-Hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors in the treatment of hypercholesterolemia. JAMA 258:3532–3536
Illingworth DR, Bacon S (1987) Hypolipidemic effects of HMG-CoA reductase inhibitors in patients with hypercholesterolemia. Am J Cardiol 60:33G–42G
Mabuchi H, Haba T, Tatami R et al (1981) Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med 305:478–482
Steinberg D (2006) The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part IV: the (1984) coronary primary prevention trial ends it–almost. J Lipid Res 47:1–14
Moride Y, Hegele RA, Langer A et al (2008) Clinical and public health assessment of benefits and risks of statins in primary prevention of coronary events: resolved and unresolved issues. Can J Cardiol 24:293–300
Neil HA, DeMicco DA, Luo D et al (2006) Analysis of efficacy and safety in patients aged 65–75 years at randomization: Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes Care 29:2378–2384
Sever PS, Poulter NR (2005) Dahlöf B et al (2005) Reduction in cardiovascular events with atorvastatin in 2, 532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care 28:1151–1157
Heart Protection Study Collaborative Group (2007) Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20, 536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 45:645–654
Knopp RH, d’Emden M, Smilde JG et al (2006) Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 29:1478–1485
Thavendiranathan P, Bagai A, Brookhart MA et al (2006) Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med 166:2307–2313
LaRosa JC, He J, Vupputuri S (1999) Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 282:2340–2346
Sever PS, Dahlöf B, Poulter NR et al (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361:1149–1158
Ridker PM, Danielson E, Fonseca FA et al (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359:2195–207
Pitt B, Waters D, Brown WV et al (1999) Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 341:70–76
LaRosa JC, Grundy SM, Waters DD et al (2005) Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352:1425–1435
Pedersen TR, Faergeman O, Kastelein JJ et al (2005) High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 294:2437–2445
Schwartz GG, Olsson AG, Ezekowitz MD et al (2001) Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285:1711–1718
Liem AH, van Boven AJ, Veeger NJ et al (2002) Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 23:1931–1937
de Lemos JA, Blazing MA, Wiviott SD et al (2004) Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 292:1307–1316
Ray KK, Cannon CP, Cairns R et al (2005) Relationship between uncontrolled risk factors and C-reactive protein levels in patients receiving standard or intensive statin therapy for acute coronary syndromes in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 46:1417–1424
Ovbiagele B, Saver JL (2007) Intensive statin therapy after stroke or transient ischemic attack: a SPARCLing success? Stroke 38:1110–1112
No authors listed (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389
Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:7–22
Arca M (2007) Atorvastatin efficacy in the prevention of cardiovascular events in patients with diabetes mellitus and/or metabolic syndrome. Drugs 67(Suppl 1):43–54
Arca M, Gaspardone A (2007) Atorvastatin efficacy in the primary and secondary prevention of cardiovascular events. Drugs 67(Suppl 1):29–42
Khush KK, Waters DD (2006) Effects of statin therapy on the development and progression of heart failure: mechanisms and clinical trials. J Card Fail 12:664–674
Investigators Gissi-Hf (2008) Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372:1231–1239
Kjekshus J, Apetrei E, Barrios V et al (2007) Rosuvastatin in older patients with systolic heart failure. N Engl J Med 357:2248–2261
Nishikawa H, Miura S, Zhang B et al (2002) Pravastatin promotes coronary collateral circulation in patients with coronary artery disease. Coron Artery Dis 13:377–381
Borghi C, Veronesi M, Prandin MG et al (2001) Statins and blood pressure regulation. Curr Hypertens Rep 3:281–288
McFarlane SI, Muniyappa R, Francisco R et al (2002) Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 87:1451–1458
Inami N, Nomura S, Shouzu A et al (2007) Effects of pitavastatin on adiponectin in patients with hyperlipidemia. Pathophysiol Haemost Thromb 36:1–8
Kinlay S, Schwartz GG, Olsson AG et al (2008) Inflammation, statin therapy, and risk of stroke after an acute coronary syndrome in the MIRACL study. Arterioscler Thromb Vasc Biol 28:142–147
Vlachopoulos C, Aznaouridis K, Dagre A et al (2007) Protective effect of atorvastatin on acute systemic inflammation-induced endothelial dysfunction in hypercholesterolaemic subjects. Eur Heart J 28:2102–2109
Blanco-Colio LM, Martín-Ventura JL, de Teresa E et al (2007) Elevated ICAM-1 and MCP-1 plasma levels in subjects at high cardiovascular risk are diminished by atorvastatin treatment. Atorvastatin on Inflammatory Markers study: a substudy of Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration. Am Heart J 153:881–888
Pignatelli P, Sanguigni V, Lenti L et al (2007) Oxidative stress-mediated platelet CD40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin. J Thromb Haemost 5:1170–1178
Hanefeld M, Marx N, Pfützner A et al (2007) Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. J Am Coll Cardiol 49:290–297
Goicoechea M, de Vinuesa SG, Lahera V et al (2006) Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. J Am Soc Nephrol 17:S231–S235
Patti G, Chello M, Pasceri V et al (2006) Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J Am Coll Cardiol 48:1560–1566
Muhlestein JB, May HT, Jensen JR et al (2006) The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J Am Coll Cardiol 48:396–401
Sola S, Mir MQ, Lerakis S et al (2006) Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol 47:332–337
Macin SM, Perna ER, Farías EF et al (2005) Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J 149:451–457
Blanco-Colio LM, Martín-Ventura JL, de Teresa E et al (2007) Increased soluble Fas plasma levels in subjects at high cardiovascular risk: Atorvastatin on Inflammatory Markers (AIM) study, a substudy of ACTFAST. Arterioscler Thromb Vasc Biol 27:168–174
Arnaud C, Braunersreuther V, Mach F (2005) Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med 15:202–206
Hennekens CH, Hollar D, Eidelman RS et al (2006) Update for primary healthcare providers: recent statin trials and revised National Cholesterol Education Program III guidelines. MedGenMed 8:54
Grundy SM, Cleeman JI, Merz CN et al (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239
Kashani A, Phillips CO, Foody JM et al (2006) Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 114:2788–2797
Shepherd J (2006) Who should receive a statin these days? Lessons from recent clinical trials. J Intern Med 260:305–319
Christopher-Stine L (2006) Statin myopathy: an update. Curr Opin Rheumatol 18:647–653
Itagaki M, Takaguri A, Kano S et al (2009) Possible mechanisms underlying statin-induced skeletal muscle toxicity in l6 fibroblasts and in rats. J Pharmacol Sci 109:94–101
Vaughan CJ, Gotto AM Jr (2004) Update on statins: 2003. Circulation 110:886–892
Bellosta S, Paoletti R, Corsini A (2004) Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 109:III50–III57
Jones P, Kafonek S, Laurora I et al (1998) Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 81:582–587
Downs JR, Clearfield M, Weis S et al (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279:1615–1622
Furberg CD, Adams HP Jr, Applegate WB et al (1994) Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 90:1679–1687
Zhao XQ, Brown BG, Hillger L et al (1993) Effects of intensive lipid-lowering therapy on the coronary arteries of asymptomatic subjects with elevated apolipoprotein B. Circulation 88:2744–2753
Blankenhorn DH, Azen SP, Kramsch DM et al (1993) Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann Intern Med 119:969–976
Waters D, Higginson L, Gladstone P et al (1995) Effects of cholesterol lowering on the progression of coronary atherosclerosis in women. A Canadian Coronary Atherosclerosis Intervention Trial (CCAIT) substudy. Circulation 92:2404–2410
Ifergan I, Wosik K, Cayrol R et al (2006) Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol 60:45–55
Lin R, Liu J, Peng N et al (2006) Lovastatin reduces apoptosis and downregulates the CD40 expression induced by TNF-alpha in cerebral vascular endothelial cells. Curr Neurovasc Res 3:41–47
Townsend KP, Shytle DR, Bai Y et al (2004) Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res 78:167–76
Greenwood J, Walters CE, Pryce G et al (2003) Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J 17:905–7
Nabatov AA, Pollakis G, Linnemann T et al (2007) Statins disrupt CCR5 and RANTES expression levels in CD4+ T lymphocytes in vitro and preferentially decrease infection of R5 versus X4 HIV-1. PLoS ONE 2:e470
Mira E, León B, Barber DF et al (2008) Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol 181:3524–3534
Sun D, Fernandes G (2003) Lovastatin inhibits bone marrow-derived dendritic cell maturation and upregulates proinflammatory cytokine production. Cell Immunol 223:52–62
Liang SL, Liu H, Zhou A (2006) Lovastatin-induced apoptosis in macrophages through the Rac1/Cdc42/JNK pathway. J Immunol 177:651–656
Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) (1998) Circulation 97:1440-1445
Shepherd J, Blauw GJ, Murphy MB et al (2002) Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360:1623–1630
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (2002) Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288:2998–3007
Nakamura H, Arakawa K, Itakura H et al (2006) Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 368:1155–1163
Tanaka T, Porter CM, Horvath-Arcidiacono JA et al (2007) Lipophilic statins suppress cytotoxicity by freshly isolated natural killer cells through modulation of granule exocytosis. Int Immunol 19:163–173
Wiesbauer F, Kaun C, Zorn G et al (2002) HMG CoA reductase inhibitors affect the fibrinolytic system of human vascular cells in vitro: a comparative study using different statins. Br J Pharmacol 135:284–292
Dimitrova Y, Dunoyer-Geindre S, Reber G et al (2003) Effects of statins on adhesion molecule expression in endothelial cells. J Thromb Haemost 1:2290–2299
Chen SD, Hu CJ, Yang DI et al (2005) Pravastatin attenuates ceramide-induced cytotoxicity in mouse cerebral endothelial cells with HIF-1 activation and VEGF upregulation. Ann N Y Acad Sci 1042:357–364
Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M et al (2005) Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 163:479–487
Abe Y, Izumi T, Urabe A et al (2006) Pravastatin prevents myocardium from ischemia-induced fibrosis by protecting vascular endothelial cells exposed to oxidative stress. Cardiovasc Drugs Ther 20:273–280
Suzuki G, Iyer V, Cimato T et al (2008) Pravastatin Improves Function in Hibernating Myocardium by Mobilizing CD133+ and cKit+ Bone Marrow Progenitor Cells and Promoting Myocytes to Reenter the Growth Phase of the Cardiac Cell Cycle. Circ Res 104(2):255–264. doi:10.1161/CIRCRESAHA.108.188730
Kwak B, Mulhaupt F, Myit S et al (2000) Statins as a newly recognized type of immunomodulator. Nat Med 6:1399–1402
Takahashi HK, Mori S, Iwagaki H et al (2005) Differential effect of LFA703, pravastatin, and fluvastatin on production of IL-18 and expression of ICAM-1 and CD40 in human monocytes. J Leukoc Biol 77:400–407
Djaldetti M, Salman H, Bergman M et al (2006) Effect of pravastatin, simvastatin and atorvastatin on the phagocytic activity of mouse peritoneal macrophages. Exp Mol Pathol 2006(80):160–164
Li Y, Köster T, Mörike C et al (2006) Pravastatin prolongs graft survival in an allogeneic rat model of orthotopic single lung transplantation. Eur J Cardiothorac Surg 30:515–524
Ji P, Si MS, Podnos Y et al (2002) Prevention of chronic rejection by pravastatin in a rat kidney transplant model. Transplantation 74:821–827
Li X, Liu C, Cui J et al (2009) Effects of pravastatin on the function of dendritic cells in patients with coronary heart disease. Basic Clin Pharmacol Toxicol 104:101–106
Kapur NK, Musunuru K (2008) Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc Health Risk Manag 4:341–353
Holdaas H, Fellström B, Jardine AG et al (2003) Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 361:2024–2031
Hedblad B, Wikstrand J, Janzon L et al (2001) Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS). Circulation 103:1721–1726
Anderssen SA, Hjelstuen AK, Hjermann I et al (2005) Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis 178:387–397
Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B, Meier B, Lescol Intervention Prevention Study (LIPS) Investigators (2002) Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 287:3215–3222
Tiefenbacher CP, Kapitza J, Dietz V et al (2003) Reduction of myocardial infarct size by fluvastatin. Am J Physiol Heart Circ Physiol 285:H59–H64
Ogata Y, Takahashi M, Takeuchi K et al (2002) Fluvastatin induces apoptosis in rat neonatal cardiac myocytes: a possible mechanism of statin-attenuated cardiac hypertrophy. J Cardiovasc Pharmacol 40:907–915
Newton CJ, Xie YX, Burgoyne CH et al (2003) Fluvastatin induces apoptosis of vascular endothelial cells: blockade by glucocorticoids. Cardiovasc Surg 11:52–60
Dunoyer-Geindre S, Dimitrova Y, Fish RJ et al (2005) Fluvastatin increases the expression of adhesion molecules, monocyte chemoattractant protein-1 and tissue factor in HUVEC stimulated by patient IgG fractions containing antiphospholipid antibodies. Thromb Haemost 93:339–345
Ohtawa M, Masuda N, Akasaka I et al (1999) Cellular uptake of fluvastatin, an inhibitor of HMG-CoA reductase, by rat cultured hepatocytes and human aortic endothelial cells. Br J Clin Pharmacol 47:383–389
Ikeda U, Shimpo M, Ohki R et al (2000) Fluvastatin inhibits matrix metalloproteinase-1 expression in human vascular endothelial cells. Hypertension 36:325–329
Xu SZ, Zhong W, Watson NM et al (2008) Fluvastatin reduces oxidative damage in human vascular endothelial cells by upregulating Bcl-2. J Thromb Haemost 6:692–700
Lopez S, Peiretti F, Bonardo B et al (2000) Effect of atorvastatin and fluvastatin on the expression of plasminogen activator inhibitor type-1 in cultured human endothelial cells. Atherosclerosis 152:359–366
Haruna Y, Morita Y, Yada T et al (2007) Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum 56:1827–1835
Seeger H, Mueck AO, Lippert TH (2000) Fluvastatin increases prostacyclin and decreases endothelin production by human umbilical vein endothelial cells. Int J Clin Pharmacol Ther 38:270–272
Wang HR, Li JJ, Huang CX et al (2005) Fluvastatin inhibits the expression of tumor necrosis factor-alpha and activation of nuclear factor-kappaB in human endothelial cells stimulated by C-reactive protein. Clin Chim Acta 353:53–60
Niwa S, Totsuka T, Hayashi S (1996) Inhibitory effect of fluvastatin, an HMG-CoA reductase inhibitor, on the expression of adhesion molecules on human monocyte cell line. Int J Immunopharmacol 18:669–675
Hillyard DZ, Jardine AG, McDonald KJ et al (2004) Fluvastatin inhibits raft dependent Fcgamma receptor signaling in human monocytes. Atherosclerosis 172:219–228
Montero MT, Hernández O, Suárez Y et al (2000) Hydroxymethylglutaryl-coenzyme A reductase inhibition stimulates caspase-1 activity and Th1-cytokine release in peripheral blood mononuclear cells. Atherosclerosis 153:303–313
Földes G, von Haehling S, Okonko DO et al (2008) Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol 124:80–85
Samson KT, Minoguchi K, Tanaka A et al (2006) Inhibitory effects of fluvastatin on cytokine and chemokine production by peripheral blood mononuclear cells in patients with allergic asthma. Clin Exp Allergy 36:475–482
Kolovou GD, Katerina A, Ioannis V et al (2008) Simvastatin: two decades in a circle. Cardiovasc Ther 26:166–178
Collins R, Armitage J, Parish S et al (2003) MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361:2005–2016
Hernández-Perera O, Pérez-Sala D, Soria E, Lamas S (2000) Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res 87:616–622
Henrich D, Seebach C, Wilhelm K et al (2007) High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res 142:13–19
Spiel AO, Mayr FB, Leitner JM et al (2008) Simvastatin and rosuvastatin mobilize Endothelial Progenitor Cells but do not prevent their acute decrease during systemic inflammation. Thromb Res 123:108–113
Xu J, Liu X, Chen J et al (2008) Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via Notch signaling pathway. Am J Physiol Cell Physiol 296(3):C535–C543. doi:10.1152/ajpcell.00310.2008
Liang YJ, Shyu KG, Wang BW et al (2008) Simvastatin inhibits C-reactive protein-induced pro-inflammatory changes in endothelial cells by decreasing mevalonate pathway products. Cardiology 110:182–190
Eccles KA, Sowden H, Porter KE et al (2008) Simvastatin alters human endothelial cell adhesion molecule expression and inhibits leukocyte adhesion under flow. Atherosclerosis 200:69–79
Jiang JL, Jiang DJ, Tang YH et al (2004) Effect of simvastatin on endothelium-dependent vaso-relaxation and endogenous nitric oxide synthase inhibitor. Acta Pharmacol Sin 25:893–901
Schaefer CA, Kuhlmann CR, Weiterer S et al (2006) Statins inhibit hypoxia-induced endothelial proliferation by preventing calcium-induced ROS formation. Atherosclerosis 185:290–296
Tang D, Park HJ, Georgescu SP et al (2006) Simvastatin potentiates tumor necrosis factor alpha-mediated apoptosis of human vascular endothelial cells via the inhibition of the geranylgeranylation of RhoA. Life Sci 79:1484–1492
Taraseviciene-Stewart L, Scerbavicius R, Choe KH et al (2006) Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 291:L668–L676
Chen H, Ren JY, Xing Y, Zhang WL, Liu X, Wu P, Wang RJ, Luo Y (2009) Short-term withdrawal of simvastatin induces endothelial dysfunction in patients with coronary artery disease: a dose-response effect dependent on endothelial nitric oxide synthase. Int J Cardiol 131:313–320
Naoum JJ, Zhang S, Woodside KJ, Song W, Guo Q, Belalcazar LM, Hunter GC (2004) Aortic eNOS expression and phosphorylation in Apo-E knockout mice: differing effects of rapamycin and simvastatin. Surgery. 136:323–328
Lee TS, Chang CC, Zhu Y et al (2004) Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation 110:1296–1302
Veillard NR, Braunersreuther V, Arnaud C et al (2006) Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis 188:51–58
Han KH, Ryu J, Hong KH et al (2005) HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation 111:1439–1447
Montecucco F, Burger F, Pelli G et al (2009) Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology (Oxford) 48(3):233–242. doi:10.1093/rheumatology/ken466
Ulivieri C, Fanigliulo D, Benati D et al (2008) Simvastatin impairs humoral and cell-mediated immunity in mice by inhibiting lymphocyte homing, T-cell activation and antigen cross-presentation. Eur J Immunol 38:2832–2844
Day AP, Bellavia S, Jones OT et al (1997) Effect of simvastatin therapy on cell membrane cholesterol content and membrane function as assessed by polymorphonuclear cell NADPH oxidase activity. Ann Clin Biochem 34:269–275
Deskur-Smielecka E, Wykretowicz A, Banaszak A et al (2000) The influence of treatment of hypercholesterolemic patients with simvastatin on plasma chemotactic activity and adherence of neutrophils. Int J Cardiol 75:85–90
Methe H, Kim JO, Kofler S et al (2005) Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol 25:1439–1445
Rudich SM, Mongini PK, Perez RV et al (1998) HMG-CoA reductase inhibitors pravastatin and simvastatin inhibit human B-lymphocyte activation. Transplant Proc 30:992–995
Zhang X, Jin J, Peng X, Ramgolam VS et al (2008) Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol 180:6988–6996
Ghittoni R, Patrussi L, Pirozzi K et al (2005) Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J 19:605–607
Arora M, Chen L, Paglia M et al (2006) Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA 103:7777–7782
Hillyard DZ, Cameron AJ, McDonald KJ et al (2004) Simvastatin inhibits lymphocyte function in normal subjects and patients with cardiovascular disease. Atherosclerosis 175:305–313
Marino F, Guasti L, Cosentino M et al (2008) Simvastatin treatment in subjects at high cardiovascular risk modulates AT1R expression on circulating monocytes and T lymphocytes. J Hypertens 26:1147–1155
Khush KK, Waters DD, Bittner V et al (2007) Effect of high-dose atorvastatin on hospitalizations for heart failure: subgroup analysis of the Treating to New Targets (TNT) study. Circulation 115:576–583
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Cannon CP, Braunwald E, McCabe CH et al (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350:1495–1504
Liao Y, Zhao H, Ogai A et al (2008) Atorvastatin slows the progression of cardiac remodeling in mice with pressure overload and inhibits epidermal growth factor receptor activation. Hypertens Res 31:335–344
Bezerra DG, Lacerda Andrade LM et al (2008) Atorvastatin attenuates cardiomyocyte loss in adult rats from protein-restricted dams. J Card Fail 14:151–160
Senthil V, Chen SN, Tsybouleva N et al (2005) Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 97:285–292
Schmechel A, Grimm M, El-Armouche A et al (2009) Treatment with atorvastatin partially protects the rat heart from harmful catecholamine effects. Cardiovasc Res 82(1):100–106
Grip O, Janciauskiene S, Lindgren S (2004) Circulating monocytes and plasma inflammatory biomarkers in active Crohn’s disease: elevated oxidized low-density lipoprotein and the anti-inflammatory effect of atorvastatin. Inflamm Bowel Dis 10:193–200
Thomas PB, Albini T, Giri RK et al (2005) The effects of atorvastatin in experimental autoimmune uveitis. Br J Ophthalmol 89:275–279
Graham KL, Lee LY, Higgins JP et al (2008) Failure of oral atorvastatin to modulate a murine model of systemic lupus erythematosus. Arthritis Rheum 58:2098–2104
Lu D, Goussev A, Chen J, Pannu P et al (2004) Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma 21:21–32
Lu D, Mahmood A, Goussev A et al (2004) Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg 101:813–821
Jung KH, Chu K, Jeong SW et al (2004) HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke 35:1744–1749
Buttmann M, Lorenz A, Weishaupt A et al (2007) Atorvastatin partially prevents an inflammatory barrier breakdown of cultured human brain endothelial cells at a pharmacologically relevant concentration. J Neurochem 102:1001–1008
Tanaka N, Katayama Y, Katsumata T et al (2007) Effects of long-term administration of HMG-CoA reductase inhibitor, atorvastatin, on stroke events and local cerebral blood flow in stroke-prone spontaneously hypertensive rats. Brain Res 1169:125–132
Zhang L, Zhang ZG, Liu XS et al (2007) The PI3K/Akt pathway mediates the neuroprotective effect of atorvastatin in extending thrombolytic therapy after embolic stroke in the rat. Arterioscler Thromb Vasc Biol 27:2470–2475
Assmus B, Urbich C, Aicher A et al (2003) HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res 92:1049–1055
Imanishi T, Hano T, Sawamura T et al (2004) Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol 31:407–413
Soehnlein O, Eskafi S, Schmeisser A et al (2004) Atorvastatin induces tissue transglutaminase in human endothelial cells. Biochem Biophys Res Commun 322:10510–10519
Wagner AH, Schwabe O, Hecker M (2002) Atorvastatin inhibition of cytokine-inducible nitric oxide synthase expression in native endothelial cells in situ. Br J Pharmacol 136:143–149
Wagner AH, Gebauer M, Güldenzoph B et al (2002) 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent inhibition of CD40 expression by atorvastatin in human endothelial cells. Arterioscler Thromb Vasc Biol 22:1784–1789
Mulhaupt F, Matter CM, Kwak BR et al (2003) Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res 59:755–766
Schönbeck U, Gerdes N, Varo N et al (2002) Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation 106:2888–2893
Zineh I, Luo X, Welder GJ et al (2006) Modulatory effects of atorvastatin on endothelial cell-derived chemokines, cytokines, and angiogenic factors. Pharmacotherapy 26:333–340
Martínez-Sales V, Vila V, Ferrando M et al (2007) Atorvastatin neutralizes the up-regulation of thrombospondin-1 induced by thrombin in human umbilical vein endothelial cells. Endothelium 14:233–238
Arnaud C, Burger F, Steffens S et al (2005) Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 25:1231–1236
Tanimoto A, Murata Y, Wang KY et al (2008) Monocyte chemoattractant protein-1 expression is enhanced by granulocyte-macrophage colony-stimulating factor via Jak2-Stat5 signaling and inhibited by atorvastatin in human monocytic U937 cells. J Biol Chem 283:4643–4651
Kofler S, Schlichting C, Jankl S et al (2008) Dual mode of HMG-CoA reductase inhibition on dendritic cell invasion. Atherosclerosis 197:105–110
Shimada K, Miyauchi K, Daida H (2004) Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench. Circulation 109:e213–e214
Dunn SE, Youssef S, Goldstein MJ et al (2006) Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med 203:401–412
Coward W, Chow SC (2006) Effect of atorvastatin on TH1 and TH2 cytokine secreting cells during T cell activation and differentiation. Atherosclerosis 186:302–309
Lawman S, Mauri C, Jury EC et al (2004) Ehrenstein MR. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J Immunol 173:7641–7646
Xu ZM, Zhao SP, Li QZ et al (2003) Atorvastatin reduces plasma MCP-1 in patients with acute coronary syndrome. Clin Chim Acta 338:17–24
Smith C, Halvorsen B, Otterdal K et al (2008) High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: down-regulatory effects of statins. Cardiovasc Res 79:195–203
McTaggart F, Buckett L, Davidson R et al (2001) Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 87:28B–32B
White CM (2002) A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol 42:963–970
Schuster H (2007) The GALAXY Program: an update on studies investigating efficacy and tolerability of rosuvastatin for reducing cardiovascular risk. Expert Rev Cardiovasc Ther 5:177–193
Ballantyne CM, Raichlen JS, Nicholls SJ et al (2008) Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation 117:2458–2466
Kim YS, Ahn Y, Hong MH et al (2007) Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. J Cardiovasc Pharmacol 49:376–383
Ikeda Y, Young LH, Lefer AM (2003) Rosuvastatin, a new HMG-CoA reductase inhibitor, protects ischemic reperfused myocardium in normocholesterolemic rats. J Cardiovasc Pharmacol 41:649–656
Laufs U, Gertz K, Dirnagl U et al (2002) Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res 942:23–30
Schäfer K, Kaiser K, Konstantinides S (2005) Rosuvastatin exerts favourable effects on thrombosis and neointimal growth in a mouse model of endothelial injury. Thromb Haemost 93:145–152
Miersch S, Sliskovic I, Raturi A et al (2007) Antioxidant and antiplatelet effects of rosuvastatin in a hamster model of prediabetes. Free Radic Biol Med 42:270–279
Desjardins F, Sekkali B, Verreth W et al (2008) Rosuvastatin increases vascular endothelial PPARgamma expression and corrects blood pressure variability in obese dyslipidaemic mice. Eur Heart J 29:128–137
Enomoto S, Sata M, Fukuda D et al (2009) Rosuvastatin prevents endothelial cell death and reduces atherosclerotic lesion formation in ApoE-deficient mice. Biomed Pharmacother 63:19–26
Pirro M, Schillaci G, Romagno PF et al (2009) Influence of Short-term Rosuvastatin Therapy on Endothelial Progenitor Cells and Endothelial Function. J Cardiovasc Pharmacol Ther 14(1):14–21
Zou MH, Wu Y (2008) AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol 35:535–545
McCarty MF (2004) AMPK activation may suppress hepatic production of C-reactive protein by stimulating nitric oxide synthase. Med Hypotheses 63:328–333
Sun W, Lee TS, Zhu M et al (2006) Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 114:2655–2662
Choi HC, Song P, Xie Z et al (2008) Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem 283:20186–20197
Li X, Han Y, Pang W et al (2008) AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28:1789–1795
Besler C, Doerries C, Giannotti G et al (2008) Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther 6:1071–1082
Prasad R, Giri S, Nath N et al (2005) Inhibition of phosphoinositide 3 kinase-Akt (protein kinase B)-nuclear factor-kappa B pathway by lovastatin limits endothelial-monocyte cell interaction. J Neurochem 94:204–214
Prinz V, Laufs U, Gertz K, Endres M et al (2008) Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke 39:433–438
Wu H, Lu D, Jiang H et al (2008) Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma 25:130–139
Wu H, Lu D, Jiang H et al (2008) Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J Neurosurg 109:691–698
Nakata S, Tsutsui M, Shimokawa H et al (2007) Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Arterioscler Thromb Vasc Biol 27:92–98
Kobayashi N, Takeshima H, Fukushima H et al (2009) Cardioprotective effects of pitavastatin on cardiac performance and remodeling in failing rat hearts. Am J Hypertens 22:176–182
Cheng CF, Juan SH, Chen JJ et al (2008) Pravastatin attenuates carboplatin-induced cardiotoxicity via inhibition of oxidative stress associated apoptosis. Apoptosis 13:883–894
Ye Y, Lin Y, Atar S et al (2006) Myocardial protection by pioglitazone, atorvastatin, and their combination: mechanisms and possible interactions. Am J Physiol Heart Circ Physiol 291:H1158–H1169
Schroeter MR, Humboldt T, Schäfer K et al (2008) Rosuvastatin reduces atherosclerotic lesions and promotes progenitor cell mobilisation and recruitment in apolipoprotein E knockout mice. Atherosclerosis doi:10.1016/j.atherosclerosis.2008.11.013
Takenouchi Y, Kobayashi T, Matsumoto T et al (2008) Possible involvement of Akt activity in endothelial dysfunction in type 2 diabetic mice. J Pharmacol Sci 106:600–608
Waiczies S, Prozorovski T, Infante-Duarte C et al (2005) Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol 174:5630–5635
Merla R, Ye Y, Lin Y et al (2007) The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am J Physiol Heart Circ Physiol 293:H1918–H1928
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Buhaescu I, Izzedine H (2007) Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 40:575–584
Narumiya S, Ishizaki T, Watanabe N (1997) Rho effectors and reorganization of actin cytoskeleton. FEBS Lett 410:68–72
Noma K, Oyama N, Liao JK (2006) Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290:C661–C668
Tramontano AF, O’Leary J, Black AD et al (2004) Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun 320:34–38
Chrissobolis S, Sobey CG (2006) Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke 37:2174–2180
Acknowledgement
This research was funded by EU FP7, Grant number 201668, AtheroRemo and supported by a grant from the Swiss National Science Foundation to Dr. F. Mach (# 310000-118245).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montecucco, F., Mach, F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin Immunopathol 31, 127–142 (2009). https://doi.org/10.1007/s00281-009-0150-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-009-0150-y