Abstract
Atherosclerosis is a chronic inflammatory disease of the arterial wall where both innate and adaptive immune responses contribute to disease initiation and progression. Recent studies established that subtypes of T cells, regulatory T cells (Tregs), actively involved in the maintenance of immunological tolerance, inhibit the development and progression of atherosclerosis. Here, we review the current knowledge on the Treg response and the major cytokines involved in its modulation in the context of atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atherosclerosis is a pathological condition of the arterial wall that underlies adverse vascular events including coronary artery disease, stroke, abdominal aortic aneurisms, and ischemic gangrene, responsible for most of the cardiovascular morbidity and mortality in the western world. Epidemiological studies also indicate that the prevalence of atherosclerosis is increasing due to the adoption of western lifestyle in developing countries and the accumulation of metabolic risk factors [1, 2].
Experimental and clinical studies provided strong evidence supporting a crucial role for inflammation in the development and progression of atherosclerosis. Atherosclerosis is initiated by focal endothelial activation in large- and medium-size arteries induced by biochemical and physical stimulation, including hypertension and hypercholesterolemia. Initially, lipoproteins infiltrate the artery wall to an extent that exceeds the capacity for elimination and are retained in the intima space [3]. Low-density lipoprotein (LDL) modifications, through enzymatic attack or non-enzymatic oxidation, release bioactive phospholipids that can activate endothelial cells. Activated endothelial cells express leukocyte adhesion molecules and release chemokines, which promote leukocyte recruitment (monocytes and lymphocytes) into the intima [4]. Monocytes become successively macrophages and foam cells after maturation and lipid phagocytosis and interact with vascular and inflammatory surrounding cells. Pathogenic T cells recognize modified autoantigens including oxidized LDL and heat shock proteins (i.e., HSP-60) that are presented by antigen-presenting cells such as macrophages or dendritic cells (DCs). The accumulation of inflammatory cells within the arterial wall leads to local production of chemokines, interleukins (IL), and proteases that enhance the influx of monocytes and lymphocytes thereby promoting the progression of atherosclerotic lesions (for review [5, 6]).
Pathogenic Th1-driven responses in atherosclerosis
Experimental studies have clearly shown that the adaptive immune system affects the development of atherosclerosis [7]. In humans, most of the T cells in atherosclerotic plaques express αβ-T cell receptor (TCR) and are of the T helper type 1 (Th1) cells. They are responsible for cell-mediated immunity and secrete interferon (IFN)-γ, IL-2, and IL-22, in contrast to Th2 cells, which secrete IL-4, IL-5, IL-10, and IL-13, and provide help for antibody production by B cells. In mice, deficiency in both T and B cells, as occurs in apolipoprotein E-deficient (apoE−/−) or LDL receptor-deficient (LDLr−/−) mice on a recombination-activating gene-deficient background, is associated with a significant reduction in early atherosclerotic lesion development [8, 9]. Moreover, atherosclerosis is enhanced after the transfer of CD4+ T cells from apoE−/− mice into apoE−/−xSCID immunodeficient mice, indicating a proatherogenic role of T cells [10]. The transplanted cells produced high levels of IFN-γ, suggesting a Th1-related proatherogenic effect. Subsequent studies have shown that cytokines or transcription factors involved in the differentiation and/or activation of Th1 cells also contribute to the atherosclerotic process. Differentiation of Th1 cells requires TCR activation by DCs. Distinct subsets of DCs elicit distinct T helper responses [11]. IL-12 production by DCs plays a critical role in Th1 differentiation as DCs from IL-12−/− mice fail to induce Th1 responses [12]. IL-12 activates the transcription factor signal transducer and activator of transcription 4 (STAT4) and a unique Th1 transcription factor, T-box expressed in T cells (T-bet), leading to upregulation of IFN-γ, the prototypic Th1 cytokine, in T cells. IL-12 synergizes with IL-18 for full induction of IFN-γ. In addition, proinflammatory mediators such as tumor necrosis factor receptor and IL-1 receptor as well as costimulatory signals, including CD40/CD40L interaction, contribute to DC maturation and induction of Th1 cells. All these proinflammatory mediators, costimulatory molecules, and transcription factors involved in Th1 differentiation and activation are expressed in atherosclerotic plaques of mice and humans and are required for initial plaque development [13–15] as well as for the perpetuation of plaque inflammation and “instability” [14–17] in mouse models of atherosclerosis. Overall, these results provide convincing elements to incriminate Th1-related responses in the promotion of plaque development and progression.
Th2-driven responses in atherosclerosis
Differentiation toward Th2 cells requires specific factors. IL-6, IL-13, and OX40-L (CD134; OX40 ligand) may play a role in DC-induced Th2 differentiation [18]. Particularly, IL-4 activates the Th2 transcription factor Gata3 through STAT6, induces IL-5, and downregulates IFN-γ. Counter-regulation between Th1 and Th2 may result from a balance between T-bet and Gata3 [19]. It has therefore been proposed that Th2-biased responses antagonize proatherogenic Th1 effects and thereby should confer atheroprotection. In fact, a number of experiments, especially those exploring the role of humoral immunity in atherosclerosis, suggest that Th2-driven humoral immune responses may be atheroprotective. A switch toward a Th2 cytokine profile in mouse models of atherosclerosis is associated with increased production of “protective” anti-oxidized LDL (oxLDL) antibodies [20]. Furthermore, splenectomy in cholesterol-fed apoE−/− mice, which is associated with reduced levels of IgM and Th2-related IgG anti-oxLDL antibodies, increase atherosclerosis [21]. Production of high titers of IgM-type anti-oxLDL antibodies, as observed following immunization of apoE−/− mice with malondialdehyde-LDL, is associated with reduced lesion size [22–24]. These antibodies arise from B1 cells and appear to be under the control of IL-5 produced by modified LDL-specific Th2 cells [25, 26]. A switch toward the production of Th2-related IgG1 antibodies has been reported in mice overexpressing IL-10, which was associated with a reduction in lesion size [27]. In addition, promoting Th2 responses in mice with mild hypercholesterolemia resulted in a reduction of early fatty-streak formation [28]. However, other data indicate that Th2 responses may be proatherogenic. Deficiency in IL-4, the prototypic Th2-related cytokine, is associated with a decrease in atherosclerotic lesion formation [29], particularly at the advanced stages of lesion progression [30]. Thus, even though initial lesion development in mice is mostly under the control of Th1-related immunity and could be counter-regulated by the promotion of a Th2 response, this may occur at the risk of favoring plaque progression as the lesion progresses with time in a hypercholesterolemic context. Therefore, the attractive concept of Th1 and Th2, yin and yang controlling the development of atherosclerosis, may not stand at all stages of plaque development. It would therefore be at risk to promote Th2 responses as a strategy to modulate atherosclerosis.
Implication of Th-17 T cells in atherosclerosis
Although T cell categorization may be attractive in its simplicity, it has become apparent that the original Th1/Th2 paradigm is much more complicated than the originally appreciated. Recently, a novel lineage of T cells, Th17, characterized by production of the inflammatory cytokine IL-17, was identified and shown to promote inflammatory autoimmune diseases including acute encephalomyelitis [31, 32]. The development of Th17 cells from naive precursor cells is potently inhibited by products of the Th1 and Th2 lineages, and promoted in vivo by IL-23 independently of the transcription factors T-bet, STAT1, STAT4, and STAT6 [31, 32]. In vitro, the induction of Th17 subset is independent of IL-23 and can be achieved by transforming growth factor (TGF-ß) in combination with IL-6 [33, 34]. IL-6 induces a STAT3-dependent IL-21 production leading to increased expression of IL-23 receptor and induction of the orphan nuclear receptor RORγt, which in synergy with STAT3, promotes IL-17 expression, and suppresses Foxp3 [35–37]. The role of Th17 in atherosclerosis development is unknown. LDLr/IL-6−/− mice, which exhibit a decrease of IL-17 [37], have a small non-significant reduction in lesion development [38], suggesting a potential minor role for Th17 in the promotion of atherogenesis. However, it is likely that the promotion of a Th17 pathway would be associated with decreased proatherogenic Th1 responses, which should ultimately be protective against lesion development. Further studies are required to elucidate the specific and direct role of Th17 in atherosclerosis.
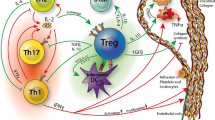
In summary, Th1 profile appears to clearly be more pathogenic than Th2 or Th17 during the development of atherosclerosis. The hypothesis that excessive activation of Th1 and/or Th2 pathways may result from alteration in regulatory immune responses has been proposed based on the discovery of a protective role for anti-inflammatory regulatory T cells (Tregs) in atherosclerosis [39, 40]. These cells have the capacity to suppress both pathogenic Th1 and Th2 responses, suggesting their important protective role against atherogenesis (cf Fig. 1).
Tregs and maintenance of self-tolerance
Natural Treg cells (nTregs), characterized by the expression of CD4, CD25, and the transcriptional factor Foxp3 (forkhead/winged helix transcription factor) develop in the thymus and recognize specific self-antigen (for review [41]). They home to peripheral tissues to maintain self-tolerance and to prevent autoimmunity by inhibiting pathogenic lymphocytes. Subsets of Treg cells, induced Treg cells (iTreg), are also generated in the periphery during an active immune response. Naive CD4+CD25 in the periphery can be converted in the presence of TGF-β, IL-10, or low dose of antigenic peptide into CD4+CD25+Foxp3+ cells. The iTreg cells can be induced by IL-10 (Tr1 cells) or TGF-β (Th3 cells). They mediate suppressor functions through the production of IL-10 and TGF-β, respectively (cf Table 1).
Under homeostatic conditions, the natural Treg cells are the major cells exhibiting suppressor functions. This population is composed of two Treg subsets that have distinct phenotypes and homeostasis in normal unmanipulated mice. One is composed of quiescent cells with long lifespan and the other is a cycling nTreg subset that divides extensively and expresses multiple activation markers, suggesting that it is composed of autoreactive Tregs that are continuously activated by tissue self-antigens [42]. An extremely close TCR clonal homology has been found between regulatory and memory CD4+ T cells, suggesting that a proportion of this regulatory population is generated from rapidly dividing, highly differentiated memory CD4+ T cells [43]. In vitro, these cells are anergic. They do not proliferate upon stimulation but are able to inhibit effector T cell proliferation [44]. The transcriptional factor, Foxp3, is necessary to mediate the inhibitory effect of Treg cells on pathogenic T cells since no suppressive function was observed in CD4+CD25+ isolated from Foxp3 knockout mice [45]. Foxp3 is specific for Treg cell lineage. However, functional Foxp3 is not required for Treg cell development [46] but is instrumental, through its interaction with other transcription factors such as nuclear factor of activated T cells [47] and acute myeloid leukemia 1 [48], in transcriptional repression of genes involved in T cell activation such as IL-2, and at the opposite, induces genes required for Treg function such as CD25 (IL-2 receptor-α chain) and CTLA-4 (cytotoxic T-lymphocyte antigen 4) [47, 49–51]. Foxp3-mediated regulation unique to the thymus more specifically affects genes encoding nuclear factors that control gene expression and chromatin remodeling. In contrast, Foxp3 target genes shared by the thymic and peripheral Treg cells encode primarily plasma membrane proteins as well as cell-signaling proteins [52].
Three general modes of suppression have been proposed to explain the inhibitory actions of Treg cells on activated T cells, although these are not completely elucidated. These include cell contact-dependent suppression, consumption and limitation of growth factors such as IL-2, and production of inhibitory cytokines.
Cell contact-dependent inhibition mediated by Treg cells involves engagement of CTLA-4 expressed on Treg cells with CD80 molecules expressed on effector T cells or interaction of CTLA-4 with CD80/CD86 on DCs [53]. The interaction between Treg cells and DCs induces indoleamine dioxygenase which leads to downregulation of effector T cell responses through tryptophan catabolism [54]. A passive mechanism of effector T cell inactivation mediated by Treg cells was also proposed. Effector T cells secrete IL-2 upon activation, which binds to CD25 on Treg cells, maintains and activates Treg cell genes such as Foxp3, which in turn downregulates IL-2 secretion in a feedback loop. Once activated, Treg cells suppress effector T cells but also deprive them from IL-2. One recent finding demonstrated that Treg cells induce apoptosis of activated T cells in vitro and in vivo by depriving them from IL-2 [55]. In addition, Treg cells could inhibit activated lymphocytes by producing soluble inhibitory cytokines such as IL-10 and TGF-β (see below) or IL-35 [56]. Production of these cytokines may also induce deactivation of DCs, leading to a loss of ability to activate effector T cells with distinct antigen specificity to Treg cells, a mechanism called bystander immune suppression. However, the requirement of these soluble cytokines in suppressive function mediated by nTreg in vivo is complicated by the existence of iTreg that use overlapping inhibition mechanisms.
Two major mediators of Treg cell function with relevance to atherosclerosis: TGF-β and IL-10
TGF-β
TGF-β and Treg cell function
The importance of TGF-β in the immune system was highlighted by the discovery that TGF-β-deficient mice develop multiple inflammatory diseases [57, 58]. These were associated with enhanced T cell proliferation, activation, and a switch of T cell differentiation toward both Th1 and Th2 profiles. The activation of T cells in this setting results from the fact that TGF-β inhibits the proliferation, activation, and differentiation of T cells towards Th1 and Th2 [59, 60]. In addition, TGF-β1 has been shown to maintain Treg cells in the periphery by acting as a costimulatory factor for expression of Foxp3 [61]. This dual effect on effector T cells and Treg cells is likely to contribute to TGF-β regulation of peripheral T cell tolerance.
Studies concerning the modalities of requirement of TGF-β for Treg-suppressive function remain controversial [62, 63]. In vitro, TGF-β produced by Treg cells appears to be dispensible for the suppressive function of Treg cells [64], but adoptive transfer of Treg cells with specific deletion of TGF-β failed to inhibit T cell-induced colitis in vivo, suggesting the importance of Treg cell-derived TGF-β in maintaining self-tolerance [64]. However, previous studies with Treg cells isolated from TGF-β1-deficient mice have generated contrasting results [65–67]. In particular, transfer of Treg cells from DO11.10.TGF-ß1−/− mice, which recognize ovalbumin epitope together with CD4+CD45RBhigh cells into lymphopenic mice, prevented colitis similarly to the equivalent cells taken from DO11.10/TGF-ß1+/+ mice, showing that Treg cells can develop in the absence of TGF-ß1 and retain suppressor function in vivo [66]. Interestingly, the suppressor function of TGF-ß1−/− Treg was abrogated by injection of anti-TGF-β, indicating that TGF-ß1 is important for the suppressive function of Treg cells even though these cells do not produce it [66].
Besides maintenance of nTreg in the periphery, several studies demonstrated that TGF-β induced conversion of Foxp3− cells to Foxp3+ cells in extrathymic sites. Overexpression of TGF-β in mouse T cells increased the proportion of Th3, which are protective in adoptive transfer model of experimental autoimmune encephalomyelitis [68]. Additional studies have extended these findings in vivo, particularly in settings of chronic antigen exposure or antigen-specific tolerance induction. For example, oral exposure to antigen induced antigen-specific Foxp3+ cells in large part dependent on TGF-β [69]. Furthermore, continuous low-dose administration of specific peptide induced a TGF-β-dependent formation of suppressor cells that can persist for long periods in the absence of antigen [70]. Thus, it appears that the induction of Foxp3+ cells in the periphery depends on TGF-β. The gut environment represents a preferential site of extrathymic Treg cell development depending on TGF-β. The intestinal immune system has evolved redundant regulatory strategies to maintain immune homeostasis. In this regard, the gut is home to a large number of Treg cells, which have the capacity to inhibit many pathogenic T cells in an antigen-independent manner using bystander suppression mechanism (for review [71]). DCs have the capacity to induce Treg cell formation depending on TGF-β and retinoic acid, which is a vitamin A metabolite highly expressed in the gut-associated lymphoid tissue [72, 73]. Recent studies proposed a mechanism that explains how DCs induce Treg cell development in the gut [74, 75]. DC expresses the integrin alphavbeta8, which has the capacity to activate the inactive form of TGF-β. This factor is secreted in inactive complexes with a latency-associated peptide, a protein derived from the N-terminal region of the TGF-β gene product, and extracellular activation of these complexes is critical for TGF-β function. It was reported that DC lacking integrin alphavbeta8 fail to induce Treg cells in vitro, an effect that depends on TGF-β activity [75]. Furthermore, these mice have reduced proportions of Treg cells in colonic tissue and, thus, increased T cell activation, leading to colitis [75]. Therefore, integrin alphavbeta8-mediated TGF-β activation by DC is essential for preventing immune dysfunction. This mechanism appears to be specific for the colon since the Treg number in the spleen of integrin alphavbeta8-knockout mice remains unaffected [75]. Thus, it is possible that DCs activate TGF-β, and in the presence of IL-6, induce the formation of Treg cells via paracrine effects.
TGF-β converts naive T cells into Treg cells that prevent autoimmunity. However, in the presence of IL-6, TGF-β also promotes the differentiation of naive T lymphocytes into cytokine-producing Th17 cells, which promote autoimmunity and inflammation [36]. This raises the question of how TGF-β can generate such distinct outcomes. Recently, Mucida et al. identified the vitamin A metabolite, retinoic acid, as a key regulator of TGF-β-dependent immune responses capable of inhibiting the IL-6-driven induction of proinflammatory Th17 cells and promoting anti-inflammatory Treg cell differentiation [72]. The authors concluded that a common metabolite such as retinoic acid can regulate the balance between pro- and anti-inflammatory pathways. However, these data were complicated by recent results showing that TGF-β in combination with IL-6 not only leads to increased IL-17 but also IL-10, which has anti-inflammatory properties [76]. These cells have regulatory effects and are protective against experimental autoimmune encephalomyelitis. Hence, TGF-β and IL-6 drive Th17 regulatory cells, which are different from pathogenic Th17 lineage induced, for example, by IL-23.
Role of T cell-dependent TGF-β in atherosclerosis
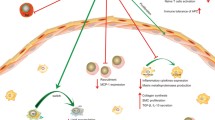
Previous studies using TGF-β neutralizing antibodies [77], soluble TGF-β receptors [78], or genetic deficiency in TGF-β [79] demonstrated an anti-atherosclerotic effect of TGF-β in apoE−/− mice. In these murine models, accelerated atherosclerosis development was observed with increased infiltration of inflammatory cells within lesions together with reduced collagen content [77, 78]. Therefore, TGF-β has anti-inflammatory effects in addition to its stabilizing effects within the lesions through the induction of extracellular matrix synthesis.
The availability of mice with specific deletion of TGF-β signaling in T cells facilitated the study of the specific role of TGF-β in T cell-induced atherosclerosis [80, 81]. The transplantation of bone marrow from T cell dominant-negative TGF-β receptor type II mice into irradiated LDLr−/− mice revealed accelerated atherogenesis [81] and increased differentiation of T cells toward both Th1 and Th2 phenotypes [80, 81]. These studies clearly showed a protective role of T cell-specific TGF-β activity against atherogenesis by inhibiting activation of both Th1 and Th2 pathways.
The cellular source of TGF-β within atherosclerotic lesions is multiple since all atheroma-associated cells have the capacity to produce this cytokine. Treg cells, which can be both source and target of TGF-β, may contribute to its production and/or protective activity. Strategies using mouse models with genetic deficiency of Treg cells or strategies using CD25 neutralizing antibodies clearly demonstrated a protective role of Treg cells against atherogenesis [39]. Moreover, Treg depletion did not influence lesion size or inflammatory phenotype when the host T cells do not respond to TGF-β, suggesting that this factor is required for the atheroprotective effect of Treg cells. Furthermore, reduction in atherosclerosis in apoE−/− mice has also been achieved through adoptive transfer of CD4+CD25+ Tregs [39, 82] (cf Fig. 1).
IL-10
IL-10 and Treg cell function
Experiments using specific deletion of IL-10 in lymphocytes have revealed the importance of this cytokine in the protection against inflammatory processes. Mice with deficiency of IL-10 are susceptible to inflammatory diseases such as colitis [83]. Furthermore, adoptive transfer of IL-10-deficient CD4+ T cells into lymphopenic mice induces severe colitis despite the ability of the recipient’s innate immune system to produce IL-10 [84]. In lymphocytes, the production of IL-10 has been associated to Th2 subset and Treg cells. Among the Tregs, both nTreg and induced Tr1 cells have the capacity to produce IL-10. Tr1 cells exhibit their suppressor function by a cell contact-independent, cytokine-dependent mechanism that involves both IL-10 and TGF-β. Several experiments have revealed the requirement of IL-10 to modulate the activation of DCs that prime Tr1 development. Specifically, it has been shown that the culture of bone marrow cells in the presence of IL-10 induces the differentiation of tolerogenic DCs expressing CD11clowCD45RBhigh, which have the capacity to induce Tr1 phenotype in vitro and in vivo [85]. However, more recent study showed that the formation of Tr1 cells expressing IL-10 rather requires the presence of TGF-β and may occur independently of IL-10 [86]. In addition, IL-27 (a member of IL-12 family) was recently involved, together with TGF-β, in promoting the generation of IL-10-producing Tr1 cells [37, 87]. We can therefore speculate that TGF-β-induced Treg cells stimulate DC to produce IL-27, which promotes Tr1 formation.
Role of IL-10 in atherosclerosis
The role of endogenous IL-10 has been clearly established in mouse models of atherosclerosis. We and others have shown that IL-10 deficiency in C57BL/6 mice fed an atherogenic cholate-containing diet promotes early atherosclerotic lesion formation characterized by increased infiltration of inflammatory cells, particularly activated T cells, and by increased production of proinflammatory cytokines [88, 89]. Similar results have been reported in IL-10−/−/apoE−/− mice fed a chow diet [90]. More recently, using a model of chimeric LDLr−/− mice in which bone marrow cells were deficient in IL-10, we showed that the absence of IL-10 induced a clear switch toward a Th1 immune response, associated with enhanced accumulation of T cells and macrophages within the lesions [91]. These results provided evidence that leukocyte-derived IL-10 is instrumental in the prevention of atherosclerotic lesion development and in the modulation of cellular and collagen plaque composition, at least in part, through a systemic immune response modulation [91]. The effect of IL-10 disruption in specific cell subtypes (macrophages, DCs, or T cells) on lesion development and progression is still unknown. Consistent with a protective role of IL-10 in atherosclerosis, systemic or local overexpression of IL-10 by adenoviral gene transfer in collar-induced carotid atherosclerosis of LDLr−/− mice was found to be highly efficient in preventing atherosclerosis [92]. It is noteworthy that overexpression of IL-10 by activated T lymphocytes reduced atherosclerosis in LDLr−/− mice [27]. The authors attributed these effects to a switch towards a Th2-like phenotype but failed to report on IL-4 production. In fact, the mouse strain used in that study has been shown to be unable to generate Th2 responses [93], leading us to suggest that the protective effect on atherosclerosis was associated with a Tr1-like phenotype. This is consistent with studies showing that transfer of clones of Tr1 cells reduces lesion development in apoE−/− mice [94], and that promotion of endogenous adaptive Tr1 cell response plays a significant role in limiting disease development during the natural course of atherosclerosis [95] (see below).
Strategies to enhance Treg function in atherosclerosis
Strategies to promote CD4+CD25+Foxp3+ Treg cell function in vivo
Following our initial report on the protective role of CD4+CD25+Foxp3+ Treg cells in atherosclerosis [39], other studies have been published supporting an anti-atherogenic role for this T cell subtype. Recent studies highlighted the role of inducible costimulatory molecule (ICOS) on Treg responses in atherosclerosis [96]. LDLr−/− mice transplanted with ICOS-deficient marrow showed accelerated atherosclerosis and enhanced infiltration of CD4+ T cells as well as increased macrophage content. This was associated with decreased numbers of Foxp3+ Treg cells and impaired in vitro Treg-suppressive function in ICOS-deficient mice compared with control mice, suggesting that ICOS modulates atherosclerosis through its effect on Treg cell responses [96]. Compound deficient apoE−/−/Cxcl10−/− mice fed a western-style diet demonstrated significant reductions in atherogenesis as compared with apoE−/− controls, and this was associated with increased Foxp3 expression, as well as IL-10 and TGF-β1 immunostaining [97]. These studies in mice seem to be relevant to the clinical situation in humans where defective Treg cell number/function has been associated with the presence of advanced stable or unstable coronary artery disease [98–100].
The identification of a critical role of naturally occurring Treg cells in atherosclerosis has led to the initiation of studies aimed at promoting Treg response in vivo. Two different strategies have been used. Based on the unique capacity of anti-CD3-specific antibodies to restore self-tolerance in type 1 diabetes [101], Steffens et al. applied this strategy in the context of atherosclerosis and showed that anti-CD3 antibody therapy reduced plaque development when administered before a high-cholesterol diet and markedly decreased lesion progression in mice with already established atherosclerosis. This was associated with increased production of TGF-β and enhanced expression of Foxp3 in lymph node and spleen cells, respectively, suggesting a regulatory immune response [102]. The other strategy consisted of the induction of oral tolerance through oral administration of oxLDL or HSP-60 to LDLr−/− mice and resulted in a significant attenuation of the initiation and progression of atherogenesis, associated with increased antigen-specific TGF-β and/or IL-10 production and increased number of CD4+CD25+Foxp3+ cells in spleen and mesenteric lymph nodes [103, 104]. Even though no direct evidence was presented to relate changes in Treg cell response to disease limitation, these studies suggest novel potential therapeutic avenues in atherosclerosis based on the modulation of Treg response.
Another strategy may consist on the interference with Treg-inhibitory signals, such as leptin-dependent signaling. Leptin directly affects the immune response, and initial studies reported reversal of starvation-induced immunosuppression in vivo following leptin administration associated with enhanced T cell proliferation and promotion of Th1 proinflammatory response [105]. More recent studies clearly showed that lack or inhibition of leptin/leptin receptor pathway protects against the development of various immunoinflammatory diseases in experimental models, ranging from colitis [106] to encephalomyelitis [107, 108] or diabetes [109]. Protection was associated, at least in some experiments [108], with a shift of the cytokine profile toward increased Th2/Treg type and increased number of Treg cells in lymphoid organs of mice with defective leptin signaling. Therefore, leptin signaling may alter Treg cell function and accelerate atherosclerosis. In agreement with this hypothesis, we recently reported [110] that leptin-deficiency (ob/ob) in LDLr−/− mice induces an unexpected 2.2- to 6-fold reduction in atherosclerotic lesion development compared with LDLr−/− mice having similar total cholesterol levels. LDLr−/−/ob/ob mice show reduced Th1 response, enhanced expression of Foxp3, the specification transcription factor of regulatory Treg cells, and improved Treg cell function. Leptin receptor-deficient (db/db) mice display marked increase in the number and suppressive function of Treg cells. Supplementation of Treg-deficient lymphocytes with Treg cells from db/db mice in apoE−/− mice induces a significant reduction of lesion size and a marked inhibition of IFN-γ production compared with supplementation by Treg cells from wild-type mice. Our results are substantiated by those of De Rosa et al. [111] showing that leptin inhibits the proliferation of Treg cells in vitro and in vivo, in part through modulation of cyclin-dependent kinase inhibitor p27 (p27(kip1)) and the phosphorylation of the extracellular-related kinases 1 (ERK1) and ERK2. These results identify a critical role for leptin/leptin receptor pathway in the modulation of the regulatory immune response and point to an important target for therapeutic interventions in immune and autoimmune diseases such as atherosclerosis.
Strategies to promote Foxp3-independent Treg response
Several studies addressed the role of mucosal tolerance to HSP (expected to induce Treg cells) in the development of experimental atherosclerosis [112, 113]. Both studies showed a reduction in lesion size following oral administration of HSP-65 in LDLr−/− mice immunized with Mycobacterium tuberculosis or fed an atherogenic diet [112, 113], suggesting that tolerance induction toward HSP may be protective against atherosclerosis. The mechanisms leading to lesion reduction have not been clearly delineated, but the T cell cytokine profile was switched toward a Th2 phenotype with high production of IL-4 [112] or IL-10 [113]. These results suggest that mucosal administration of antigen reduces plaque development. However, additional mechanistic work is required to understand the potential role of the regulatory immune response in this process.
The hypothesis that an imbalance exists between the effector and the regulatory arms of the immune response suggests that supplementation with Treg cells may lead to the induction of immune suppression and a reduction in pathogenic T cell-mediated responses, ultimately altering plaque development and/or composition. We showed that the administration of a clone of ovalbumin-specific Tr1 cells [114], with its cognate antigen, to apoE−/− mice, induced a significant suppression of Th1 (and Th2)-mediated responses and led to an increase in IL-10 production by stimulated peripheral T cells [94]. Tr1 responses were associated with a significant reduction in atherosclerotic plaque development and a marked reduction in the relative accumulation of inflammatory macrophages and T lymphocytes with a preservation of smooth muscle cell and collagen contents of the atherosclerotic plaques. These results showed that modulation of the peripheral immune response is achievable by transfer of Tr1 cells with no specificity to a known plaque antigen and leads to limitation of plaque development in apoE−/− mice, probably through bystander immune suppression. Whether this could be achieved with a Tr1-clone specific to a plaque-derived antigen remains to be addressed. More recently, we showed that treatment of apoE−/− mice with measles virus nucleoprotein, a component of measles virus known to inhibit DC activation [115], induces a DC-dependent Tr1-like phenotype characterized by increased IL-10 but reduced IFN-γ and IL-4 production, and leads to significant inhibition of lesion development and progression [116]. These results suggest that immunomodulatory properties of measles virus may be harnessed for the treatment of atherosclerosis. However, it will be important to examine whether this could be obtained without significant side effects.
Conclusion
The last decade has witnessed major advances in our understanding of the pathophysiology of atherosclerosis. The discovery of endogenous counter-regulators of the pathogenic immune response in atherosclerosis led to the identification of an important role for Treg cells in the control of lesion development and/or progression. Efforts should be directed toward the delineation of the major determinants of the regulatory response, the critical subtypes of Treg cells responsible for these protective effects, and the molecular mechanisms involved in their survival, migration, homing, and suppressive function. It is also of utmost importance to examine the therapeutic potential of Treg cells specific for plaque-derived antigens or that of vaccination-like strategies using such antigens to promote a disease-specific regulatory response and reduce disease development and complications.
References
Bonow RO (2002) Primary prevention of cardiovascular disease: a call to action. Circulation 106(25):3140–3141. doi:10.1161/01.CIR.0000048067.86569.E1
Lopez AD, Murray CC (1998) The global burden of disease, 1990–2020. Nat Med 4(11):1241–1243. doi:10.1038/3218
Skalen K, Gustafsson M, Rydberg EK et al (2002) Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417(6890):750–754. doi:10.1038/nature00804
Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L (2001) Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med 194(2):205–218. doi:10.1084/jem.194.2.205
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695. doi:10.1056/NEJMra043430
Tedgui A, Mallat Z (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86(2):515–581. doi:10.1152/physrev.00024.2005
Binder CJ, Chang MK, Shaw PX et al (2002) Innate and acquired immunity in atherogenesis. Nat Med 8(11):1218–1226. doi:10.1038/nm1102-1218
Dansky HM, Charlton SA, Harper MM, Smith JD (1997) T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A 94(9):4642–4646. doi:10.1073/pnas.94.9.4642
Daugherty A, Pure E, Delfel-Butteiger D et al (1997) The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J Clin Invest 100(6):1575–1580. doi:10.1172/JCI119681
Zhou X, Nicoletti A, Elhage R, Hansson GK (2000) Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102(24):2919–2922
Banchereau J, Briere F, Caux C et al (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi:10.1146/annurev.immunol.18.1.767
Maldonado-Lopez R, De Smedt T, Michel P et al (1999) CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med 189(3):587–592. doi:10.1084/jem.189.3.587
Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P (1998) Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394(6689):200–203. doi:10.1038/28204
de Nooijer R, von der Thusen JH, Verkleij CJ et al (2004) Overexpression of IL-18 decreases intimal collagen content and promotes a vulnerable plaque phenotype in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol 24(12):2313–2319. doi:10.1161/01.ATV.0000147126.99529.0a
Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH (2005) T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A 102(5):1596–1601. doi:10.1073/pnas.0409015102
Mallat Z, Corbaz A, Scoazec A et al (2001) Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 89(7):E41–E45. doi:10.1161/hh1901.098735
Schonbeck U, Sukhova GK, Shimizu K, Mach F, Libby P (2000) Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A 97(13):7458–7463. doi:10.1073/pnas.97.13.7458
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673):245–252. doi:10.1038/32588
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100(6):655–669. doi:10.1016/S0092-8674(00)80702-3
Zhou X, Paulsson G, Stemme S, Hansson GK (1998) Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest 101(8):1717–1725. doi:10.1172/JCI1216
Caligiuri G, Nicoletti A, Poirier B, Hansson GK (2002) Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109(6):745–753
Palinski W, Miller E, Witztum JL (1995) Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A 92(3):821–825. doi:10.1073/pnas.92.3.821
Freigang S, Horkko S, Miller E, Witztum JL, Palinski W (1998) Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol 18(12):1972–1982
George J, Afek A, Gilburd B et al (1998) Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 138(1):147–152. doi:10.1016/S0021-9150(98)00015-X
Binder CJ, Hartvigsen K, Chang MK et al (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 114(3):427–437
Binder CJ, Shaw PX, Chang MK et al (2005) The role of natural antibodies in atherogenesis. J Lipid Res 46(7):1353–1363. doi:10.1194/jlr.R500005-JLR200
Pinderski LJ, Fischbein MP, Subbanagounder G et al (2002) Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res 90(10):1064–1071. doi:10.1161/01.RES.0000018941.10726.FA
Huber SA, Sakkinen P, David C, Newell MK, Tracy RP (2001) T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 103(21):2610–2616
King VL, Szilvassy SJ, Daugherty A (2002) Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol 22(3):456–461. doi:10.1161/hq0302.104905
Davenport P, Tipping PG (2003) The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol 163(3):1117–1125
Harrington LE, Hatton RD, Mangan PR et al (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6(11):1123–1132. doi:10.1038/ni1254
Park H, Li Z, Yang XO et al (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6(11):1133–1141. doi:10.1038/ni1261
Bettelli E, Carrier Y, Gao W et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238. doi:10.1038/nature04753
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24(2):179–189. doi:10.1016/j.immuni.2006.01.001
Zhou L, Ivanov II, Spolski R et al (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8(9):967–974. doi:10.1038/ni1488
Nurieva R, Yang XO, Martinez G et al (2007) Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448(7152):480–483. doi:10.1038/nature05969
Korn T, Bettelli E, Gao W et al (2007) IL-21 initiates an alternative pathway to induce proinflammatory T(H) 17 cells. Nature 448(7152):484–487. doi:10.1038/nature05970
Song L, Schindler C (2004) IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis 177(1):43–51. doi:10.1016/j.atherosclerosis.2004.06.018
Ait-Oufella H, Salomon BL, Potteaux S et al (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12(2):178–180. doi:10.1038/nm1343
Mallat Z, Ait-Oufella H, Tedgui A (2007) Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med 17(4):113–118. doi:10.1016/j.tcm.2007.03.001
Stephens GL, Shevach EM (2007) Foxp3+ regulatory T cells: selfishness under scrutiny. Immunity 27(3):417–419. doi:10.1016/j.immuni.2007.08.008
Fisson S, Darrasse-Jeze G, Litvinova E et al (2003) Continuous activation of autoreactive CD4+CD25+ regulatory T cells in the steady state. J Exp Med 198(5):737–746. doi:10.1084/jem.20030686
Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC (2007) The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol 7(3):231–237. doi:10.1038/nri2037
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY (2005) Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22(3):329–341. doi:10.1016/j.immuni.2005.01.016
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4(4):330–336. doi:10.1038/ni904
Lin W, Haribhai D, Relland LM et al (2007) Regulatory T cell development in the absence of functional Foxp3. Nat Immunol 8(4):359–368. doi:10.1038/ni1445
Wu Y, Borde M, Heissmeyer V et al (2006) FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126(2):375–387. doi:10.1016/j.cell.2006.05.042
Ono M, Yaguchi H, Ohkura N et al (2007) Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 446(7136):685–689. doi:10.1038/nature05673
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299(5609):1057–1061. doi:10.1126/science.1079490
Williams LM, Rudensky AY (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 8(3):277–284. doi:10.1038/ni1437
Marson A, Kretschmer K, Frampton GM et al (2007) Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445(7130):931–935. doi:10.1038/nature05478
Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY (2007) Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445(7130):936–940. doi:10.1038/nature05563
Paust S, Lu L, McCarty N, Cantor H (2004) Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A 101(28):10398–10403. doi:10.1073/pnas.0403342101
Fallarino F, Grohmann U, Hwang KW et al (2003) Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 4(12):1206–1212. doi:10.1038/ni1003
Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ (2007) CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8(12):1353–1362. doi:10.1038/ni1536
Collison LW, Workman CJ, Kuo TT et al (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569. doi:10.1038/nature06306
Kulkarni AB, Karlsson S (1993) Transforming growth factor-beta-1 knockout mice–a mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143:3–9
Shull MM, Ormsby I, Kier AB et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699. doi:10.1038/359693a0
Li MO, Sanjabi S, Flavell RA (2006) Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25(3):455–471. doi:10.1016/j.immuni.2006.07.011
Marie JC, Liggitt D, Rudensky AY (2006) Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25(3):441–454. doi:10.1016/j.immuni.2006.07.012
Cobbold SP, Castejon R, Adams E et al (2004) Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol 172(10):6003–6010
Nakamura K, Kitani A, Strober W (2001) Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194(5):629–644. doi:10.1084/jem.194.5.629
Piccirillo CA, Letterio JJ, Thornton AM et al (2002) CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med 196(2):237–246. doi:10.1084/jem.20020590
Li MO, Wan YY, Flavell RA (2007) T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26(5):579–591. doi:10.1016/j.immuni.2007.03.014
Kullberg MC, Hay V, Cheever AW et al (2005) TGF-beta1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol 35(10):2886–2895. doi:10.1002/eji.200526106
Fahlen L, Read S, Gorelik L et al (2005) T cells that cannot respond to TGF-beta escape control by CD4+CD25+ regulatory T cells. J Exp Med 201(5):737–746. doi:10.1084/jem.20040685
Nakamura K, Kitani A, Fuss I et al (2004) TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol 172(2):834–842
Carrier Y, Yuan J, Kuchroo VK, Weiner HL (2007) Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol 178(1):179–185
Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA (2005) Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 115(7):1923–1933. doi:10.1172/JCI24487
Apostolou I, von Boehmer H (2004) In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199(10):1401–1408. doi:10.1084/jem.20040249
Belkaid Y (2007) Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol 7(11):875–888. doi:10.1038/nri2189
Mucida D, Park Y, Kim G et al (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317(5835):256–260. doi:10.1126/science.1145697
Sun CM, Hall JA, Blank RB et al (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204(8):1775–1785. doi:10.1084/jem.20070602
Lacy-Hulbert A, Smith AM, Tissire H et al (2007) Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A 104(40):15823–15828. doi:10.1073/pnas.0707421104
Travis MA, Reizis B, Melton AC et al (2007) Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449(7160):361–365. doi:10.1038/nature06110
McGeachy MJ, Bak-Jensen KS, Chen Y et al (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8(12):1390–1397. doi:10.1038/ni1539
Mallat Z, Gojova A, Marchiol-Fournigault C et al (2001) Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res 89(10):930–934. doi:10.1161/hh2201.099415
Lutgens E, Gijbels M, Smook M et al (2002) Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol 22(6):975–982. doi:10.1161/01.ATV.0000019729.39500.2F
Grainger DJ, Mosedale DE, Metcalfe JC, Bottinger EP (2000) Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci 113(Pt 13):2355–2361
Gojova A, Brun V, Esposito B et al (2003) Specific abrogation of transforming growth factor-beta signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood 102:4052–4058
Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK (2003) Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest 112(9):1342–1350
Mor A, Planer D, Luboshits G et al (2007) Role of naturally occurring CD4+CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol 27(4):893–900. doi:10.1161/01.ATV.0000259365.31469.89
Davidson NJ, Leach MW, Fort MM et al (1996) T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184(1):241–251. doi:10.1084/jem.184.1.241
Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190(7):995–1004. doi:10.1084/jem.190.7.995
Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H (2003) Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18(5):605–617. doi:10.1016/S1074-7613(03)00113-4
Maynard CL, Harrington LE, Janowski KM et al (2007) Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol 8(9):931–941. doi:10.1038/ni1504
Stumhofer JS, Silver JS, Laurence A et al (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8(12):1363–1371. doi:10.1038/ni1537
Mallat Z, Besnard S, Duriez M et al (1999) Protective role of interleukin-10 in atherosclerosis. Circ Res 85(8):e17–e24
Pinderski Oslund LJ, Hedrick CC, Olvera T et al (1999) Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol 19(12):2847–2853 in process citation
Caligiuri G, Rudling M, Ollivier V et al (2003) Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 9(1–2):10–17
Potteaux S, Esposito B, Van Oostrom O et al (2004) Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 24:1474–1478
Von Der Thusen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA (2001) Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J 15(14):2730–2732
Hagenbaugh A, Sharma S, Dubinett SM et al (1997) Altered immune responses in interleukin 10 transgenic mice. J Exp Med 185(12):2101–2110. doi:10.1084/jem.185.12.2101
Mallat Z, Gojova A, Brun V et al (2003) Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 108(10):1232–1237. doi:10.1161/01.CIR.0000089083.61317.A1
Ait-Oufella H, Horvat B, Kerdiles Y et al (2007) Measles virus nucleoprotein induces a regulatory immune response and reduces atherosclerosis in mice. Circulation 116(15):1707–1713. doi:10.1161/CIRCULATIONAHA.107.699470
Gotsman I, Grabie N, Gupta R et al (2006) Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation 114(19):2047–2055. doi:10.1161/CIRCULATIONAHA.106.633263
Heller EA, Liu E, Tager AM et al (2006) Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113(19):2301–2312. doi:10.1161/CIRCULATIONAHA.105.605121
Mor A, Luboshits G, Planer D, Keren G, George J (2006) Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur Heart J 27(21):2530–2537. doi:10.1093/eurheartj/ehl222
de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC (2007) Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE 2(1):e779. doi:10.1371/journal.pone.0000779
Han SF, Liu P, Zhang W et al (2007) The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol 124(1):90–97. doi:10.1016/j.clim.2007.03.546
Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L (2003) TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9(9):1202–1208. doi:10.1038/nm924
Steffens S, Burger F, Pelli G et al (2006) Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation 114(18):1977–1984. doi:10.1161/CIRCULATIONAHA.106.627430
van Puijvelde GH, Hauer AD, de Vos P et al (2006) Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation 114(18):1968–1976. doi:10.1161/CIRCULATIONAHA.106.615609
van Puijvelde GH, van Es T, van Wanrooij EJ et al (2007) Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol 27(12):2677–2683. doi:10.1161/ATVBAHA.107.151274
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394(6696):897–901. doi:10.1038/29795
Siegmund B, Sennello JA, Jones-Carson J et al (2004) Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut 53(7):965–972. doi:10.1136/gut.2003.027136
Matarese G, Di Giacomo A, Sanna V et al (2001) Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol 166(10):5909–5916
De Rosa V, Procaccini C, La Cava A et al (2006) Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest 116(2):447–455. doi:10.1172/JCI26523
Lee CH, Chen YG, Chen J et al (2006) Novel leptin receptor mutation in NOD/LtJ mice suppresses type 1 diabetes progression: II. Immunologic analysis. Diabetes 55(1):171–178. doi:10.2337/diabetes.55.01.06.db05-1129
Taleb S, Herbin O, Ait-Oufella H et al (2007) Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol 27:2691–2698
De Rosa V, Procaccini C, Cali G et al (2007) A key role of leptin in the control of regulatory T cell proliferation. Immunity 26(2):241–255. doi:10.1016/j.immuni.2007.01.011
Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J (2002) Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol 40(7):1333–1338. doi:10.1016/S0735-1097(02)02135-6
Maron R, Sukhova G, Faria AM et al (2002) Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation 106(13):1708–1715. doi:10.1161/01.CIR.0000029750.99462.30
Groux H, O’Garra A, Bigler M et al (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389(6652):737–742. doi:10.1038/39614
Lemarie CA, Esposito B, Tedgui A, Lehoux S (2003) Pressure-induced vascular activation of nuclear factor-kappaB: role in cell survival. Circ Res 93(3):207–212. doi:10.1161/01.RES.0000086942.13523.88
Ait-Oufella H, Kinugawa K, Zoll J et al (2007) Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115(16):2168–2177. doi:10.1161/CIRCULATIONAHA.106.662080
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ait-Oufella, H., Taleb, S., Mallat, Z. et al. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol 31, 23–33 (2009). https://doi.org/10.1007/s00281-009-0143-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-009-0143-x