Abstract
The recent identification of cytosolic pattern recognition receptors (PRRs) with leucine-rich repeats, which recognize pathogen-associated molecular patterns (PAMPs), has been garnering considerable attention. Activated PRRs form molecular complexes called inflammasomes, consisting of related proteins that include procaspase 1[interleukin (IL) 1β converting enzyme (ICE)]. Inflammasomes have been shown to facilitate molecular proximity, stimulate activation of procaspase 1, which consequently produces inflammatory cytokines IL-1β and IL-18 and ultimately lead to the initiation of innate immunity. An adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC), which recruits PRRs carrying the pyrin homologous domain (PYD) and procaspase 1 through PYD and CARD, respectively, is responsible for the formation of some inflammasomes and also activation of procaspase 1. In this review, our main attention will be directed to PYD region analysis of ASC to understand the interaction between PYD-carrying PRRs and ASC. Taking into consideration the other aspects of the ASC gene in the proapoptotic ability and down-regulation by methylation, the biological function of ASC will be discussed in relation to the epigenetic aspects of infection, inflammation, and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cytosolic receptors different form from TLRs in detecting pathogens-associated molecular patterns
The host has systems that respond rapidly to diverse groups of microorganisms and pathogens, including intracellular metabolites. Recognition of pathogen-associated molecular patterns (PAMPs) is achieved by membrane-type or cytosolic pattern-recognition receptors (PRRs) containing leucine-rich repeats (LRRs). Of these PRRs, toll-like receptors (TLRs) have been most intensively investigated as membrane-type receptors for the last decade. TLRs detect PAMPs, such as lipopolysaccharides (LPS) of gram-negative bacteria, flagellin, and bacterial and viral nucleic-acid motifs [1], thereby inducing various intracellular signaling cascades for the initiation of the innate immune response.
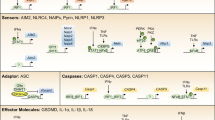
Recently, it has been established that PAMPs are also recognized by a PRR family of cytosolic leucine-rich receptors called nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). NLRs include proteins such as NOD1, NOD2 [2], NACHT (NOD)-, LRR- and Pyrin-domain-containing proteins (PYD; NALPs)-carrying PRRs, interleukin (IL)-1β converting enzyme (ICE)-protease activating factor (IPAF), and neuronal apoptosis inhibitor proteins (NAIPs) [3]. Cytosolic NLRs induce signaling cascades for initiation of innate immune responses as has been seen in TLRs [4]. Additionally, Inohara et al. reported that NOD proteins recognize peptidoglycan-derived molecules, leading to activation of NF-κB [5–7].
Cytosolic leucine-rich NOD like receptors and IL-1β and IL-18 production
Another recent topic is that some NLRs recognize exogenously and/or endogenously derived pathogenic factors [8], leading to activation of 45 kD procaspase-1 (previously well known as ICE) into 10P (10 kD) and 20P (20 kD) forms through the assembly of a cytosolic protein complex called the inflammasome [9]. Inflammasomes then induce molecular proximity advantageous to activation of procaspase 1. Active caspase-1 is required for the processing and subsequent release of immature proinflammatory cytokines, such as prointerleukin 1β (proIL-1β) and proIL-18 to IL-1β and IL-18. In addition to IL-1β and IL-18, it has been reported that IL-33, which is involved in generating a T helper 2-cell response to allergies, is also produced from immature form cleavage by caspase-1 [10].
Inflammasome and Caspase 1 activation
While proIL-1β is induced through NF-kB activation transduced by TLRs and NOD proteins, much attention has been directed to determine the mechanisms that regulate maturation of proIL-1β to its active form, IL-1β and secretion. As previously mentioned, biologically active IL-1β and IL-18 are generated by cleavage of proforms of these cytokines by caspase-1 [11], although the entire mechanism of how IL-1β and IL-18 are released from cells still remains unclear. In some cases, it seems to involve release of the entire inflammasome complex [9]. Attempts to clarify the components of various types of inflammasomes, with special attention to NLRs, are currently being done.
Involvement of ASC with NLR, NALP3, and inflammasome formation
Of the NLRs, considerable attention has been given to NALP3 (Cryopyrin), whose mutation is associated with several hereditary periodic syndromes including Muckle–Wells syndrome, familial cold urticaria, and chronic infantile neurological cutaneous and articular syndrome [12–14], to clarify its recognition of PAMPs and the mechanism of inflammasome formation. It has been reported that various pathogens, toxins, bacterial RNA, and gout crystals trigger NALP3 inflammasomes by inducing a conformational change in NALP3 by an unknown mechanism, leading to exposure of the NACHT (NOD) domain and NALP3 oligomerization. Following this, NALP3 interacts through homotypic protein–protein interactions of pyrin domains (PYDs) with apoptosis-associated speck-like protein containing a CARD (ASC), a central adaptor within the inflammasome [15]. In addition to a PYD, ASC also contains a caspase recruitment domain (CARD), which is a key domain in recruiting procaspase-1.
Second signal of caspase 1 activation responsible for maturation of IL-1β and IL-18
According to in vitro studies, the second signal involved in caspase-1 activation and IL-1β release can be derived from the activation of purinergic receptors of the P2X7 subtype by the ligand adenosine triphosphate (ATP). P2X7-receptor-deficient mice have been shown to be completely defective in IL-1β release in response to exogeneous ATP [16]. Although the physiological relevance of ATP-mediated IL-1β release remains unknown, it was reported that cells under certain stressful conditions secrete ATP into the extra-cellular space, and activated P2X7 receptor [16] induces potassium efflux and plasma-membrane depolarization. It has also been suggested that the depletion of intracellular potassium results in activation of caspase-1 [17, 18]. For the secretion of activated IL-1β, other molecules that cause membrane blebbing and pores also appear to be required [19]. For instance, the activation of calcium-independent phospholipase A2 in association with potassium depletion was reported be a necessary factor for IL-1β secretion [20].
The adaptor protein ASC is responsible for inflammasome formation and caspase 1 activation: discovery and characteristics of ASC
As one of the critical factors in the formation of inflammasomes, the adapter protein ASC functions as a mediator to recruit NLPs and procaspase 1. The groupf of Tschopp [9] first reported that ASC is indispensable for the activation of procaspase 1. In THP1 cells, they identified a caspase-activating complex called an inflammasome, comprised of caspase-1, caspase-5, ASC, and NALP1 (DEAFCAP), a PYD-containing protein sharing structural homology with NODs. By using a cell-free system, they showed that proinflammatory caspase 1 activation and proIL-1β processing is lost upon prior immunodepletion of ASC; moreover, expression of a dominant-negative form of ASC in THP-1 cells blocks proIL-1β maturation and activation of inflammatory caspases induced by LPS in vitro.
ASC, which has been recognized as an adaptor protein responsible both for forming apoptosomes and inflammasomes, was originally found and identified as a molecule, which formed a speck near the nucleus of HL-60 cells in association with apoptosis induced by various reagents during the search for molecules responsible for altering the nuclear morphology of HL-60 cells. According to its cDNA sequence, ASC has a C-terminal CARD and a PYD [21]. Pyrin is known as a causative gene for familial Mediterranean fever [22]. From its structural characteristics, we intuitively assumed that ASC must be involved in both apoptosis and inflammation. Afterwards, we then reported on PYD homotypic interactions to form filaments [23] and later determined the responsible amino acid regions for this [24].
According to immunohistological experiments, ASC was not expressed in the basal layer of the epidermis, but expression became extensive towards the outer layers [25], where cells are exposed to external factors and destined to die. This was also the case in the bowel. Such an expression pattern led us to assume that ASC functions as not only a surveyor of pathogenic invaders, but also as an inducer of programmed cell death. Furthermore, ASC expression by monocytes and neutrophils was induced in a region of inflammation not usually characterized by ASC detection, indicating that ASC is involved in inflammation as well. Apoptotic inducers, such as Fas ligands, also induced the expression of ASC [26]. Taken together, we first speculated that ASC was related both to inflammation and apoptosis (Fig. 1), which has since been established and recognized. After we reported ASC in 1999, a speedy gene hunt for the protein family containing the pyrin homologous domain and LRR was done, and the functions of some gene products in innate immunity were established.
Hypothetical functions of ASC. The NALPs (PYD-carrying PRRs) contained not only PYD, but also leucine-rich regions, which are receptors for pathogenic molecules similar to those seen in TLR. Conceptually, PYD-carrying PRRs bound to pathogenic molecules are activated to react with ASC, thus inducing molecular proximity. The aggregates formed by molecular proximity are called inflammasomes. Procaspase 1 is also recruited to the inflammasome and activated to produce mature IL-1β, IL-18 and IL-33. Just afterwards, or simultaneously to, these reactions, ASC has also been suggested to induce apoptosis by recruiting caspase 8 and/or Bax. Thus, ASC functions in cells damaged by infection or other accidents to produce cytokines as a sign of danger to the host, likely leading them to programmed cell death
To certify that ASC really is responsible for activating caspase 1 in vivo as well as in vitro, we generated ASC-deficient mice and analyzed their phenotypes [27]. We observed that in macrophages of the peritoneal cavity and kupffer cells of the liver, no activation of caspase 1 occurred and thereby no activation of proIL-1β or proIL-18. Thus, the mice were resistant to lipopolysaccharide-induced endotoxin shock [27]. Consistent results using ASC-deficient mice were reported independently by Mariathasan et al. [28].
Endogenous inhibitors of caspase 1
Because IL-1β plays a crucial role in septic shock, it is rational to presume that there are numerous endogenous inhibitors of IL-1β activity. IL-1β -receptor antagonists inhibit IL-1β by binding the receptor without stimulating the intrinsic signal [29]. Proteinase inhibitor 9 [30], caspase-12 [31] and CARD 8 [32], all have a suppressive effect on caspase-1. Intact pyrin inhibits caspase-1 activation, while mutated pyrin, as identified in familial Mediterranean fever (FMF), cannot inhibit caspase-1 activation and consequent IL-1β release [33–35]. There seems to be two mechanisms that inhibit caspase 1 activation by pyrin products. One mechanism might involve direct interaction of the amino-terminal death domain (DD) of pyrin with the intracellular adaptor ASC proteins, which then inhibits caspase-1 activation. In addition, the carboxyl-terminal B30.2 domain of pyrin was recently proven to bind to the catalytic subunits(p20, p10) of caspase-1 [36]. It is intriguing that a large percentage of FMF-associated pyrin mutations reside in the B30.2 domain [36]; whereas these mutated pyrins might induce chronic inflammation, resistance to some infections should increase owing to an augmented innate immune response. Furthermore, several viral-negative regulators of caspase-1 activation have been described [37, 38]. In one case, the Myxoma virus encodes for a PYD-containing protein, which interacts with ASC to inhibit caspase-1 [38].
3D structure of the PYD of ASC showing an additional death domain resembling DD, DED, and CARD
Because the N terminal half (1–90 amino acids) of ASC is highly homologous to the N terminus of pyrin, we named it the pyrin-like domain (PYD) [21]. Thereafter, the PYD motif was found in more than 30 human and virus genes in silico and established as a new family within the DD superfamily based on a convincing combination of sequence alignment, functional association, structure prediction, and homology modeling [9, 39–42].The DD superfamily, which is composed of six-helices, includes the DD, death effector domain (DED), and CARD families, and mediate the crucial interactions in apoptotic and inflammatory signaling pathways. Death domains function as protein–protein interaction motifs, serving as adaptors for assembling components of signaling complexes. A classic example of this is the DD of Fas, which recruits FADD through interactions with its DD and subsequently recruits procaspase 8 through interactions of its DED domain to form the death-inducing signaling complex. Death domains only associate with their own family members, forming both homo and heteromeric associations, but strictly form homotypic interactions. The structures of two DD heterodimers indicate that at least two different binding interfaces are possible, and models based on these structures suggest that both interfaces may be used simultaneously in a trimeric complex [43–45].
The three-dimensional PYD structures of ASC and NALP1 were determined by magnetic resonance imaging (MRI) spectroscopy [46, 47], revealing close structural similarity to DDs, DEDs, and CARDs. The structural alignment with other members of the DD superfamily differed from previously predicted amino acid sequence alignments, however. Although the ASC PYD region was a very close fit to the six-helix-fold model characteristic for the DD superfamily (Fig. 2), the structure of the NALP1 PYD differed from all other known DD superfamily structures in that the helix 3 was replaced by a flexibly disordered loop. As helix 3 participated in homotypic interactions with the other members of the DD superfamily, it is surprising to us and likely significant for the function of NALP 1.
Three dimensional PYD structure and critical amino acids of ASC(quotated from [24]). We analyzed the three-dimensional structure of PYD in collaboration with Dr. Otting at Australia University. By including the results of mutational experiments, we could identify the responsible amino acids for PYD–PYD homotypic interaction; Basic K (lysine) and R (arginine) on helix 2 and D (aspartic acid) on helix 4 proved to form a critical region
The three-dimensional structures determined by MRI also suggested that two highly positively and negatively charged surfaces in ASC PYDs result in a strong electrostatic dipole moments, which are predicted to be present in related PYRIN domains as well.
Role of charged and hydrophobic residues in the oligomerization of the PYD of ASC
There are many predictions for the mode of PYD–PYD interactions described above, but no experimental evidence with MRI has been available because PYD–PYD complexes produced biochemically form highly insoluble aggregates in physiological conditions (e.g. neutral pH), thus making it impossible to use them for MRI spectroscopic measurement (Fig. 2).
Overexpression of ASC PYD in cells has been shown to result in filamentous structures in the cytoplasm through self-oligomerization [21]. Such cytoplasmic filament-like structures have been observed for many domains of the DD superfamily [48–51]; for example, overexpression of the DEDs of FADD and caspase-8 results in cytoplasmic filaments, and the CARDs of caspase-2 and mE10 have also been shown to form intracellular filaments through homotypic interactions. It is thought that filament formation reflects the physiological homotypic interactions of each DD fold. To assess the role of charged and hydrophobic residues in the oligomerization of ASC PYDs, a series of alanine-point mutants were made, and effects on ASC filament formation in ASC cDNA-transfected cells were examined. In addition, oligomerization-deficient alanine mutants of charged residues were further replaced with different charged or polar residues [24].
Hydrophobic residues that were critical for the formation of ASC PYD filaments were highly conserved among PYD family proteins. Most of the critical hydrophobic residues were buried inside of the PYD structures and thus, may play a crucial role in maintenance of the three-dimensional fold. The alanine-point mutants in Leu 25 (helix 2) and Pro40 (helix 3), which were partly facing the solvent outside of the PYD structure, showed deficiency in filament formation, suggesting an important role of Leu25 (helix 2), Pro40 (helix 3), and Met47 (helix 3) in interactions at the PYD–PYD interface.
Mutation experiments with charged residues revealed that the charges of Lys21 (helix2), Lys 26 (helix 2), and Arg41 (helix 3) and of Asp48 (helix 4) and Asp51 (helix 4) were critical for oligomerization of ASC PYDs. In addition, MRI analysis showed that Arg41 (helix 3) resides in proximity of Lys21 and Lys26 (helix 2). Thus, we currently propose that electrostatic forces between the positive charge cluster of helices 2 and 3 and negative charge cluster of helix 3 are critical for oligomerization of ASC PYD and possibly also for ASC interactions with the other PYD family proteins. It is also likely that the hydrophobic resides of Leu25 (helix 2), Pro40 (helix3), and Met47 (helix 3) stabilize PYD–PYD interactions formed by the charged residues. As MRI predicted the possible interactions between the positive charges of helices 2 and 3 and negative charges of helices 1 and 4, the mutational data based on the filament formation of ASC PYDs are mostly consistent with predictions from previous MRI studies [46, 24].
Arg42Trp pyrin mutants are associated with familial Mediterranean fever (FMF)
The positive charge of Arg41 in ASC PYDs has been predicted to be essential for PYD–PYD interactions by MRI and mutation studies [46, 24]. FMF is caused by point mutations in pyrin. One of these FMF-associated mutants, Arg42Trp, is located in the PYD of pyrin [52] and corresponds to Arg41 in ASC. In the context of the ASC PYDs, the Arg42Trp mutation was found to abolish filament formation [24]. It would be intriguing to see if the molecular mechanism behind the FMF. Arg42Trp mutation was based on the disruption of interactions between the PYDs of pyrin and ASC. This interaction is known to be of functional importance, as pyrin interacts with ASC to inhibit ASC-mediated caspase-1 activation. Notably, however, the sequence conservation between the ASC PYD and pyrin is limited. For example, of the five charged residues found to be essential for filament formation of ASC PYDs, only two of them were conserved in pyrin. Furthermore, mutation of the non-conserved residues Lys21, Asp48, and Asp51 in ASC to the oppositely charged residues Glu, Arg, and Lys, respectively, which are found in pyrin, resulted in abolishment of filament formation. Point mutations of ASC PYD were thus not sufficient to stimulate the ASC-binding function of pyrin. A more thorough mutational study of the PYD of pyrin is required to shed light on the inhibitory role of these oppositely charged pyrin residues.
Concluding Remarks
Recently, cytosolic PRRs, containing leucine-rich repeats, have been identified and discovered to detect PAMPs. PRRs are then activated to form inflammasomes, which stimulate maturation of procaspase 1 and proIL-1β, proIL-18, and proIL-33 and lead to initiation of innate immunity and apoptosis of macrophage cells [53]. The inflammasome consists of cytosolic PRRs, caspase 1, and adaptor protein ASC, the latter recruiting procaspase 1 and other PRRs through PYD and CARD. It remains to be clarified which NLRs specifically recognize which PAMPs, and which NLRs does ASC specifically interact with besides NALP3 (Table 1). A year after our first report, ASC was also reported to act as a proapoptotic and tumor suppressor gene down-regulated by methylation [54]. We confirmed methylation of the ASC gene in human melanoma, colon cancer, and lung cancer, and its down-regulation correlated with the degree of methylation in CpG islands [55, 56]. They reported the dominant negative mutant for caspase 9 but not caspase 8 inhibitts the apoptosis induced by overexpressing ASC in human embryonic kidney (HIK)293 cells(54). On the other hand, a dominant negative mutant for caspase 8 but not caspase 9 inhibits the apoptosis induced by the formed oligomerization of ASC [57]. ASC was further reported to unexpectedly react with Bax and function as a promoter of apoptosis [58]. Recently, it was reported that ASC activated the cleavage of Bid by caspase 8 and induced Bax-dependent apoptosis, and that the suppression of Bid reduced the ASC-mediated apoptosis [59]. So far, although no tumorigenesis is evident in ASC-deficient mice, chemical carcinogenesis and infection experiments are underway using ASC-deficient mice with APC- and p53-deficient mice.
Thus, taking the biological functions and the epigenetic aspects of ASC together, ASC appears be a useful probe to understand the mutual relationships among aging, innate immunity, inflammation, and cancer.
References
Akira S, Takeda K (2004) Toll-like receptor signaling. Nat Rev Immunol 4:499–511
Inohara N, Chamaillard M, McDonald C, Nunez G (2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355–383
Ting JP, Kastner DL, Hoffman HM (2006) CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol 6:183–195
Philpott DJ, Girardin SE (2004) The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 41:1099–1108
Strober W, Murray PJ, Kitani A, Watanabe T (2006) Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6:9–20
Kufer TA, Fritz JH, Philpott DJ (2005) NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol 13:381–388
Viala J et al Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nature Immunol 5:1166–1174
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442:39–44
Martinon F, Burns K, Tschopp J (2002) The nflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol Cell 10:417–426
Schmitz J. et al (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490
Thornberry NA, Molineaux SM (1995) Interleukin-1β converting enzyme: a novel cysteine protease required for IL-1β production and implicated in programmed cell death. Protein Sci 4:3–12
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat Genet 29:301–305
McDermott MF, Aksentijevich I (2002) The autoinflammatory syndromes. Curr Opin Allergy Clin Immunol 2:511–516
Feldmann J et al (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71:198–203
Martinon F, Tschopp J (2004) Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117:561–574
Solle M et al (2001) Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem 276:125–132
Perregaux D, Gabel CA (1994) Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203
Walev I, Reske K, Palmer M, Valeva A, Bhakdi S (1995) Potassium-inhibited processing of IL-1β in human monocytes. EMBO J 14:1607–1614
Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG (2006) Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145
Andrei C et al (2004) Phospholipases C and A2 control lysosome-mediated IL-1β secretion: implications for inflammatory processes. Proc Natl Acad Sci USA 101:9745–9750
Masumoto J, Taniguchi S, Ayukawa K et al (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 274(33):835–838
The International FMF Consortium (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 90:797–807
Masumoto J, Taniguchi S, Sagara J (2001) Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem Biophys Res Commun 280:652–655
Moriya M, Taniguchi S, Wu P, Liepinsh E, Otting G, Sagara J (2005) Role of charged and hydrophobic residues in the oligomerization of the PYRIN domain of ASC. Biochemistry 44:575–583
Masumoto J, Taniguchi S, Nakayama J et al (2001) Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J Histochem Cytochem 49:1269–1275
Shiohara M, Taniguchi S, Masumoto J et al (2002) ASC, which is composed of a PYD and a CARD, is up-regulated by inflammation and apoptosis in human neutrophils. Biochem Biophys Res Commun 293:1314–1318
Yamamoto M, Yaginuma K, Tsutsui H et al (2004) ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9:1055–1067
Mariathasan S, Newton K, Monack DM et al (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218
Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–2147
Annand RR et al (1999) Caspase-1 (interleukin-1β-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem J 342:655–665
Saleh M et al (2006) Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440:1064–1068
Razmara M et al (2002) CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem 277:13952–13958
Aksentijevich I et al (1999) Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am J Hum Genet 64:949–962
Chae JJ et al (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell 11:591–604
Inohara N, Nunez G (2003) NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3(5):371–82 (Review, May)
Chae JJ et al (2006) The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc Natl Acad Sci USA 103:9982–9987
Messud-Petit F et al (1998) Serp2, an inhibitor of the interleukin-1β -converting enzyme, is critical in the pathobiology of myxoma virus. J Virol 72:7830–7839
Johnston JB et al (2005) A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23:587–598
Bertin J, DiStefano PS (2000) The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ 7:1273–1274
Fairbrother WJ, Gordon NC, Humke EW, O’Rourke KM, Starovasnik MA, Yin JP, Dixit VM (2001) The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci 10:1911–1918
Pawlowski K, Pio F, Chu Z, Reed JC, Godzik A (2001) PAAD—a new protein domain associated with apoptosis, cancer and autoimmune diseases. Trends Biochem Sci 26:85–87
Staub, E., Dahl, E. and Rosenthal, A. (2001) The DAPIN family: a novel domain links apoptotic and interferon response proteins. Trends Biochem Sci 26:83–85
Xiao T, Towb P, Wasserman SA, Sprang SR (1999) Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell 99:545–555
Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y (1999) Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399:549–557
Weber CH, Vincenz C (2001) The death domain superfamily: a tale of two interfaces? Trends Biochem Sci 26:475–481
Liepinish E, Barbals R, Dahl E, Sharipo A, Staub E, Otting G (2003) The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J Mol Biol 332:1155–1163
Hiller S, Kohl A, Fiorito F, Herrmann T, Wider G, Tschopp J, Grütter MG, Wüthrich K (2003) NMR structure of the apoptosis- and inflammation-related NALP1 pyrin domain. Structure 11:1199–1205
Siegel RM, Martin DA, Zheng L, Ng S, Bertin J, Cohen J, Lenardo MJ (1998) Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol 141:1243–1253
Perez D, White E (1998) E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol 141:1255–1266
Yan M, Lee J, Schilbach S, Godard A, Dexit V (1999) mE10, a novel caspase recruitment domain-containing proapoptotic molecule. J Biol Chem 274:10287–10292
Guiet C, Vito P (2000) Caspase recruitment domain (CARD)-dependent cytoplasmic filaments mediate Bcl 10-induced NF-κB activation. J Cell Biol 148:1131–1139
Hull KM, Shoham N, Chae JJ, Aksentijevich I, Kastner DL (2003) The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol 15:61–69
Mariathasan S, Monack DM (2007) Inflammasome adaptors and sensors:intracellular regulatetors of infection and inflammation. Nat Immunol 70:31–40
Conway KE, McConnell BB, Bowring CE, Donald CD, Warren ST, Vertino PM (2000) TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res 60:6236–42
Guan X, Sagara J, Yokoyama T et al (2003) ASC/TMS1, a caspase-1 activating adaptor, is downregulated by aberrant methylation in human melanoma. Int J Cancer 107:202–208
Machida EO, Brock MV, Hooker CM, Nakayama J, Ishida A, Amano J, Picchi MA, Belinsky SA, Herman JG, Taniguchi S, Baylin SB (2006) Hypermethylation of ASC/TMS1 is a sputum marker for late-stage lung cancer. Cancer Res 66(12):6210–6218 (Jun 15)
Masumoto J, Dowds TA, Schaner P, Chen FF, Ogura Y, Li M et al (2003) ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun 303:69–73
Ohtsuka T, Ryu H, Minamishima YA et al (2004) ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol 6:121–128
Hasegawa M, Kawase K, Inohara N, Imamura R, Yeh W-C, Kinoshita T, Suda T (2007) Mechanism of ASC-mediated apoptosis:Bid-dependent apoptosis in type II cells. Oncogene 26:1748–1756
Grenier JM et al (2002) Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett 530:73–78
Wang L et al (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J Biol Chem 277:29874–29880
Mariathasan S, Weiss DS, Dixit VM, Monack DM (2005) Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. Exp Med 202:1043–1049
Gavrilin MA et al (2006) Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc Natl Acad Sci USA 103:141–146
Zamboni DS et al (2006) The Birc1e cytosolic patternrecognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature Immunol 7:318–325
Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2:e18
Molofsky AB et al (2006) Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203:1093–1104
Mariathasan S. et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232
Kanneganti TD et al (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233–236
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Acknowledgements
We thank our collaborators and all graduate students who have been involved in the study of ASC. This work was partially supported by a grant-in-aid from Novartis Pharma K.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taniguchi, S., Sagara, J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Semin Immunopathol 29, 231–238 (2007). https://doi.org/10.1007/s00281-007-0082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-007-0082-3
Keywords
- Pattern recognition receptors (PRRs)
- Leucine-rich repeats (LRRs)
- Pathogen-associated molecular patterns (PAMPs)
- A PRR family of cytosolic NOD-like receptors (NLRs)
- Apoptosis-associated speck-like protein containing a CARD (ASC)
- Pyrin homologous domain (PYD)
- NACHT-, LRR- and PYD-containing proteins (NALPs)
- Caspase 1
- Inflammasome
- IL-1β
- IL-18
- IL-33
- Apoptosis