Abstract
Background
Although substantial evidence has shown that the mammalian target of rapamycin (mTOR) pathway is an important therapeutic target in gastric cancer, the overall response rates in patients to mTOR inhibitor everolimus have been less than initially expected. We hypothesized that the limited efficacy of everolimus in gastric cancer is due to the activation of extracellular signal-regulated kinase (ERK).
Methods
ERK activation was investigated using western blot. The effects of dual inhibition of ERK and mTOR via genetic and pharmacological approaches were determined using cellular assays and xenograft mouse model.
Results
We observed the decreased phosphorylation of mTOR, rS6, and 4EBP1 and increased phosphorylation of ERK and p90RSK in gastric cancer cells exposed to everolimus at clinically relevant concentration. Using both in vitro cell culture assays and in vivo xenograft mouse model, we found that trametinib overcame everolimus resistance by either effectively targeting resistant cells or further enhancing everolimus’ efficacy in sensitive cells. Mechanism studies confirmed that trametinib overcame everolimus resistance via specifically inhibiting ERK and regulating ERK-mediated Bcl-2 family proteins in gastric cancer cells.
Conclusions
Inhibition of mTOR pathway can induce “paradoxical” activation of ERK in gastric cancer, and this activation can be reversed by trametinib. Since both drugs are clinically available, our findings might accelerate the initiation of clinical trials on gastric cancer using everolimus and trametinib combination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer, particularly prevalent in Asia, ranks fourth in cancer incidence and is the second leading cause of cancer mortality worldwide [1]. The 90% of gastric cancer cases are adenocarcinoma with intestinal and diffuse types [2]. Most patients with gastric cancer are diagnosed at advanced stage with poor prognosis [3]. In recent years, treatment options for advanced gastric cancer include molecular targeted, cellular immune and antibody-based therapies [4]. Ramucirumab, inhibitor of human epidermal growth factor receptor 2 (HER2), in combination with paclitaxel has demonstrated significant improvements in progression-free and overall survival, compared with chemotherapy alone, in patients with metastatic gastric cancer [5]. Molecular targeted therapy has shown potential in the treatment of gastric cancer at advanced stage.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that presents in two cellular complexes, mTORC1 and mTORC2, with distinct substrates and mechanisms of activation [6]. mTORC1 phosphorylates S6 kinase (S6K) and eukaryotic initiation factor-4E-binding protein (4EBP1) to enhance the translation of a subset of mRNAs; the main substrates of mTORC2 are AKT and related kinases [7]. mTOR has been identified as a novel therapeutic target in cancer due to its important roles in regulating cell growth, protein synthesis, ribosome biogenesis and lipid production [7]. Several cancers exhibit constitutive mTORC1 signaling and can be treated with sirolimus and its analog everolimus that selectively and effectively suppresses mTOR1 activity [8]. Everolimus has been approved for the treatment for breast cancer and renal cell carcinoma [9,10,11]. However, clinical trials on the efficacy of everolimus alone or its combination with standard treatment in advanced gastric cancer demonstrated that everolimus was not effective in improving the overall survival of patients with advanced gastric cancer [12, 13]. Substantial evidence has shown that mTOR inhibition induces activation of various oncogenic pathways, such as MEK/ERK, Akt, and eIF4E, which comprise the anti-cancer effect of mTOR inhibitors [14, 15]. Single-agent-targeting mTOR triggers the activation of the MEK/ERK compensatory pathway in primary prostate cancer cells. Consistently, combination of MEK and mTOR inhibitors significantly reduced prostate tumor growth in a patient-derived xenograft (PDX) mouse model [16].
We hypothesized that the limited efficacy of everolimus in gastric cancer is due to the activation of alternative oncogenic pathways, such as MEK/ERK. The present study sought to examine the impact of everolimus treatment on ERK pathway. We also investigated whether the ERK inhibition by FDA-approved MEK inhibitor trametinib can overcome everolimus resistance in gastric cancer.
Materials and methods
Cells, drugs, and viability assay
Human gastric cancer cell lines SNU1, SNU16, AGS, MKN45, and NCI-N87 were purchased from American Type Culture Collection and authenticated via short tandem repeat profiling. The molecular characteristics on the mutation of KRAS, PIK3CA, BRAF, and TP53 of these cell lines are summarized in Supplementary Table 1. Cells were cultured using minimal essential media (MEM) supplemented with 10% fetal bovine serum (Hyclone, UK), 2 mM l-glutamine, and 1% penicillin/streptomycin (Invitrogen, USA) at 37 °C in a humidified 5% CO2 atmosphere. Trametinib and everolimus (Selleckchem Inc., USA) were reconstituted in DMSO. Cell viability was determined using CellTiter® 96 Aqueous One Solution cell proliferation assay (Promega, USA). 10,000 cells/well were seeded in 96-well plate. The next day, drugs at different concentrations were added to the medium. After 3 days of treatment, cell viability was assessed according to the manufactures instructions.
Generation of everolimus-resistant cell line
AGS-r cells were established by culturing AGS parental cells (AGS-s) in the above medium with everolimus and without 1% penicillin/streptomycin. Cells were initially cultured with 50 nM everolimus for 2 weeks. The concentration of everolimus was gradually increased by twofold change each time until reaching 1.6 μM. The next dose was given only when the cells were stable in proliferation without significant death. After 8 months of culturing in the presence of increasing concentrations of everolimus, AGS-r cells were established and maintained in the medium containing 1.6 μM of everolimus.
Proliferation and apoptosis assays
10,000 cells/well were seeded in 96-well plate for proliferation assay and 100 000 cells/well were seeded in six-well plate for apoptosis assay. The next day, drugs at different concentrations were added to the medium. After 3 days of treatment, cell proliferation was measured using 5-bromo-2′-deoxyuridine (BrdU) Proliferation Assay Kit (Cell Signaling, USA). This kit uses an anti-BrdU antibody to detect BrdU incorporation into cellular DNA during cell proliferation. For apoptosis, cells were harvested using trypsin and then stained with Annexin V-FITC/7-AAD Apoptosis Kit (BD Pharmingen, US) according to the manufacturer’s instructions. Apoptotic cells were analysis on a Beckman Coulter FC500. The percentage of Annexin V-positive cells was determined by CXP software analysis.
Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot (WB) analyses
Proteins were extracted in RIPA buffer supplemented with 1 × protease inhibitor cocktail and phosphatase inhibitor (Life Technologies Inc, USA). The supernatant was collected and protein concentration was determined by the DC Protein Assay Kit (Bio-Rad, USA). Equal amount of proteins was resolved using denaturing SDS-PAGE and then processed for WB using designated primary and secondary antibodies. Antibodies include anti-p-ERK (T202/Y204), anti-ERK, anti-p-p90RSK (T359/S363), anti-p90RSK, anti-mTOR (S2481), anti-mTOR, anti-p-rS6 (S235/236), anti-rS6, anti-p-4EBP1 (T37/46), anti-4EBP1, and anti-β-actin. All antibodies except anti-β-actin (Santa Cruz Biotechnology, USA) were purchased from Cell Signaling Inc. USA. Targeted proteins were detected by Enhanced Chemiluminescence Kits (Amersham Biosciences, UK).
siRNA knockdown
Specific knockdown of human ERK1/2 was achieved by transfecting cells with siRNA which was designed according to NCBI Reference Sequences (GenBank: ERK1: NM_002746.2 and ERK2: NM_002745.4). Control and ERK1/2 siRNAs were synthesized in Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The siRNA sequences were as follows: ERK1, 5′-GAC CGG AUG UUA ACC UUU A-3′; ERK2, 5′-CCA GGA UAC AGA UCU UAA A-3′; and negative control, 5-UUC UCC GAA CGU GUC ACG U-3. 50,000 cells/well were seeded in 24-well plate. The transfection was performed using Lipofectamine 2000 (Invitrogen, USA) with a final siRNA concentration of 50 nM when cells reached 70% confluency. The transfection procedure was conducted according to the manufacture’s instructions. Reagent was removed at 6 h post-transfection and the cells were harvested for analysis of cellular activity and molecular biochemistry at 48 h post-transfection.
Gastric cancer xenograft mouse models and immunohistochemistry
Six- to eight-week-old SCID mice were used for mice studies. 10 million AGS-r or AGS-s cells were suspended in 100 μl PBS and subcutaneously injected into flank of each mouse. When tumor volume reached approximately 100 mm3, the mice were divided into different drug treatment groups (n = 10 each). Mice were given vehicle (20%/80% DMSO/saline), oral trametinib at 10 mg/kg, oral everolimus at 5 mg/kg, or the combination of trametinib and everolimus. Tumour length and width were monitored using a caliper and volumes were calculated using the formula: length × width2 × 0.5236. After 3 weeks, mice were euthanized using CO2. Tumors were isolated, fixed with 10% formalin (Sigma, USA) and stored as tissue wax block. Tumor section slides were used for cleaved caspase 3 and Ki67 staining. After dewax, rehydration and antigen retrieval, the slides were incubated with 1:500 dilution of cleaved caspase 3 or Ki67 antibody (Cell Signaling, USA) at 4 °C overnight and secondary antibody at room temperature for 2 h. The signal was developed using Pierce DAB (3,3-diaminobenzidine) Substrate kit. Quantification of apoptotic and proliferating tumor cells were performed using Image J software.
Statistical analyses
The data are expressed as mean and standard deviation. For in vitro studies, statistical analyses were performed by unpaired Student’s t test. For in vivo studies, each data point shows the mean detected value from ten different mice. One-way analysis of variance (ANOVA) with post hoc Tukey was performed to show changes in tumor size. p value < 0.05 was considered statistically significant.
Results
Everolimus induces ERK activation in gastric cancer cells
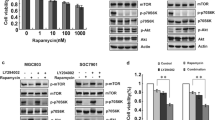
As shown in Fig. 1a, there was a decreased phosphorylation of essential molecules involved in mTOR pathway including mTOR, rS6, and 4EBP1 in gastric cancer cells at 6-, 12-, and 24-h exposure of everolimus at clinically relevant concentration. In addition, increased phosphorylation of ERK and its downstream molecule p90RSK was observed (Fig. 1a). Two representative cell lines AGS and N87 modeling gastric cancer disease with different cellular origins and genetic profiling were used in this study. Of note, the AGS cell line was derived from gastric adenocarcinoma from a patient who had received no prior therapy [17]. Our results suggest a rapid activation of ERK and inhibition of mTOR by everolimus in gastric cancer cells, and this is time dependent.
Activation of ERK occurs after everolimus treatment in gastric cancer cells. a Representative WB shows ERK and mTOR signalling in AGS and N87 cells after 200 nM everolimus treatment at various time points. b In vitro sensitivity of AGS-s and AGS-r cells to clinically relevant doses of everolimus. Cell viability was measured after 3 days treatment. c Representative WB shows ERK signaling in AGS-r and AGS-s cells. Results shown were obtained from at least three independent experiments. *p < 0.05, compared to control
To determine the long-term effect of everolimus on ERK pathway, everolimus-resistant AGS cell line (AGS-r) was generated by culturing parental AGS-s cells in the presence of everolimus at gradually increasing concentrations. Everolimus up to 1.6 μM did not affect viability of AGS-r cells whereas resulted in a decreased viability in AGS-s cells by ~ 70% (Fig. 1b). The IC50 of everolimus in AGS-r cells was ~ ten-fold higher than that in AGS-s cells. Consistent with our previous findings, there was an increased phosphorylation of ERK and p90RSK in AGS-r cells compared to AGS-s cells (Fig. 1c). These results indicate that everolimus induces ERK activation in gastric cancer cells.
Trametinib overcomes gastric cancer cell resistance to everolimus in vitro
Trametinib, a selective and effective MEK inhibitor, has been FDA approved as a single-agent for the treatment of patients with V600E-mutated metastatic melanoma [18]. To determine whether ERK activation plays a critical role in the development of everolimus resistance in gastric cancer, we used two approaches to address this hypothesis. The first approach was to investigate whether trametinib is active against AGS-r cells. As shown in Fig. 2a, b, trametinib significantly inhibited proliferation and induced apoptosis in AGS-r cells. In the second approach, we investigated the combinatory effects of trametinib and everolimus in everolimus-sensitive gastric cancer cells. We treated AGS-s cells to trametinib or everolimus alone at concentration that led to 20–50% inhibition and compared the efficacy of the combination with single drug alone. The combination of trametinib and everolimus resulted in greater efficacy than single drug alone in all tested gastric cancer cell lines, and this combinatory effect was dose dependent (Fig. 2c, d). It is noted that the combination achieved almost complete inhibition of growth and induction of apoptosis in AGS, SNU1, and SNU16 cells. The results clearly demonstrate that trametinib can overcome everolimus resistance by directly acting on resistant cells or further enhancing everolimus’ efficacy in sensitive gastric cancer cells.
MEK inhibitor trametinib increases gastric cancer cell sensitivity to everolimus in vitro. Trametinib at 100 to 500 nM significantly inhibits growth (A) and induces apoptosis (B) in AGS-r cells in a dose-dependent manner. Trametinib at 250 nM significantly enhances the anti-proliferative (C) and pro-apoptotic (D) effects of everolimus in gastric cancer cells. Cell proliferation and apoptosis were determined after 3 days of drug treatment. Results shown were obtained from at least three independent experiments. *p < 0.05, compared to control or everolimus alone
Trametinib overcomes gastric cancer cell resistance to everolimus in vivo
Next, we evaluated the in vivo effects of trametinib alone and its combination with everolimus in two independent gastric cancer xenograft models: everolimus-resistant using AGS-r cells and everolimus-sensitive using AGS-s cells. As shown in Fig. 3a, trametinib at 3 mg/kg was orally administrated daily when AGS-r xenograft tumors reached ~ 100 mm3. During 3 weeks of trametinib treatment, the mean tumor size in trametinib-treated group was significantly smaller than the vehicle group (Fig. 3a), suggesting that trametinib is effective against AGS-r growth in vivo. In addition, trametinib significantly augmented the in vivo efficacy of everolimus in inhibiting AGS-s growth in mice (Fig. 3b). The HE staining also revealed a marked decrease in tumor size in combination group compared to single drug group (Supplementary Fig. 1). Immunohistochemistry analysis of proliferation marker Ki67 and apoptosis marker cleaved caspase 3 demonstrated more apoptotic cells and less proliferating cells in the combination group compared to single drug group (Fig. 4). These results are consistent with the in vitro findings, further confirming the ability of trametinib in sensitizing gastric cancer to everolimus treatment. Of note, there was no significant body weight loss in the combination group compared to control (Fig. 3c). The mice appearance (e.g., skin and fur) and behaviors seemed to be normal. All these suggest that mice well tolerated the drug treatment.
MEK inhibitor trametinib increases gastric cancer cell sensitivity to everolimus in vivo. a AGS-r tumor growth in mice treated with trametinib and vehicle alone. 10 mg/kg of trametinib was given daily by oral gavage. b AGS-s tumor growth in mice treated with trametinib or everolimus alone, vehicle, or the combination of everolimus and trametinib. Ten mice were in each group. *p < 0.05, compared to control or everolimus alone
Effect of trametinib and everolimus in vivo in inhibiting tumor growth and inducing apoptosis. a Representative photos of tumor sections demonstrate the proliferating (labelled by Ki67) and apoptotic (labelled by cleaved caspase 3) tumor cells. The nuclei were stained with hematoxylin. Scale bar presents 50 μM. b Cleaved caspase 3 staining shows more apoptotic cells in combination arm. c Ki67 staining shows fewer proliferating cells in combination arm. Results shown are the fold change relative to control. *p < 0.05, compared to everolimus alone
Trametinib overcomes everolimus resistance in gastric cancer cells via inhibiting ERK
To investigate whether trametinib overcomes everolimus resistance in gastric cancer cells via inhibiting ERK signaling, we examined the phosphorylation level of ERK and p90RSK in gastric cancer cells treated with trametinib at a concentration that inhibits proliferation and survival of both AGS-s and AGS-r cells. We found that trametinib remarkably decreased the phosphorylation of ERK and its downstream effector p90RSK in both sensitive and resistance gastric cancer cells (Fig. 5a). In contrast, trametinib did not affect phosphorylation of JNK (Fig. 5a), suggesting that trametinib preferentially inhibits ERK signaling. MEK/ERK regulates activity and expression of Bcl-2 protein family to promote cancer cell survival [19]. Consistent with the inhibition of ERK signaling, we observed the increased Bim level in trametinib-treated AGS-r cells (Fig. 5a). In addition, trametinib decreased the level of Bcl-2, a pro-survival molecule member of Bcl-2 family. Consistently, we further found that everolimus increased the phosphorylation of ERK and p90RSK and this stimulatory effect was reversed by trametinib (Fig. 5b), demonstrating that trametinib abolished everolimus-induced ERK activation in gastric cancer cells.
Trametinib specifically inhibits ERK signaling in gastric cancer cells. a Representative WB image shows ERK signaling, Bim and Bcl-2 in AGS-s and AGS-r cells exposed to trametinib. b Trametinib inhibits everolimus-induced ERK activation in N87 cells. WB analyses were performed after 24 h of drug treatment. 250 nM trametinib and 200 nM everolimus were used. c Representative WB image shows decreased p-ERK and total ERK in AGS-r cells transfected with ERK siRNA. Proliferation (d) and apoptosis (e) of AGS-r cells after ERK depletion. Cell proliferation and apoptosis were analyzed at 5 days post-transfection. Results shown were obtained from at least three independent experiments. *p < 0.05, compared to Ctrl siRNA
To confirm the stimulatory role of ERK in everolimus-resistant gastric cancer cells, we depleted ERK using the specific ERK siRNA in AGS-r cells. As expected, we observed the minimal level of phosphorylated ERK and total ERK in AGS-r cells transfected with ERK siRNA (Fig. 5c). In addition, a significant decrease in growth and increase in cell apoptosis were detected in ERK-depleted AGS-r cells. Taken together, these results demonstrate that trametinib overcomes everolimus resistance in gastric cancer cells via inhibiting ERK.
Discussion
Studies on the thousands of gastric cancer tissues and matched normal counterparts demonstrate that mTOR activation is frequently increased in gastric cancer, and the overexpression of phosphorylated mTOR is an independent predictor of survival that negatively correlates with prognosis [20]. mTOR inhibitors, including temsirolimus and everolimus, either alone or in combination with other anticancer agents, are under evaluation in many cancers including gastric cancer [21]. Everolimus has proved to have an anticancer activity in preclinical and clinical phase I/II studies in patients with metastatic gastric cancer [22,23,24]. Unfortunately, a phase III clinical study on advanced gastric cancer patients who failed previous chemotherapy treatment demonstrated that everolimus monotherapy did not significantly improve the overall survival [12]. One explanation is that everolimus mediates its effects by inhibiting mTORC1 with limited effect on mTORC2 activity [8]. However, one recent study identified a subset of diffuse-type gastric cancer as responder to inhibitor of mTORC1 [25]. Our work demonstrates that the activation of ERK pathway contributes to everolimus resistance in gastric cancer cells, and this activation can be reversed by the addition of trametinib.
We demonstrated a rapid inhibition of mTOR and paradoxical activation of ERK by everolimus in gastric cancer cells derived from treatment-naïve patient [17] (Fig. 1a). We observed the same phenomenon in gastric cancer cells after prolonged exposure to everolimus (Fig. 1b, c), suggesting that ERK activation is a persistent feature in gastric cancer cells after everolimus treatment. ERK activity favors the adaptation to environmental stresses that are often induced by chemotherapy and anti-tumor activity of the host immune system and, therefore, ERK activation is observed in tumor cells exposed to chemotherapy [26]. Paradoxical activation of ERK is also observed in hepatocellular carcinoma cells after BRAF inhibition using sorafenib or specific BRAF/CRAF siRNA [27]. Both our findings and the previous work suggest that ERK activation plays an important role in the development of tumor cell resistance to multiple anti-cancer agents. In addition, it is important to understand whether the resistance is irreversible (due to genetic factors such as gene mutations) or reversible (due to plasticity of signaling). We speculate that multiple molecular mechanisms might contribute to everolimus resistance in gastric cancer, and ERK activation is the essential one. Future work would be determining if the AGS-r cells remain resistant to everolimus following the removal of everolimus from culture conditions for 8–12 weeks.
We further investigated whether trametinib can overcome everolimus resistance in gastric cancer. Trametinib is an oral selective MEK inhibitor with potent anti-cancer activity in various cancers that acts by inhibiting MEK1 and MEK2. It has been FDA approved in combination with dabrafenib for the treatment of patients with metastatic melanoma and non-small cell lung cancer harboring BRAFV600E mutation [28]. We found that trametinib was effective against everolimus-resistant gastric cancer cells, and its combination with everolimus resulted in greater efficacy than everolimus alone (Figs. 2, 3, 4). The molecular characteristics (e.g., mutation of PI3K, BRAF, KRAS, and TP53) of gastric cancer cell lines we selected to demonstrate the combinatory effects are summarized in Supplementary Table 1 [29, 30]. We did not observe the correlation of oncogenic genes’ mutation with cell response to combination treatment. This suggests that the combination of everolimus and trametinib is superior to everolimus alone in gastric cancer cells regardless of differential molecular profiling. Of note, mice tolerate well to the combination treatment. These results demonstrate that the co-administration of trametinib and everolimus is effective in overcoming everolimus resistance in gastric cancer with minimal toxicity in mice.
Mechanism studies showed that trametinib decreased the phosphorylation of ERK and 90RSK but not JNK in both sensitive and resistant gastric cancer cells, and abolished everolimus-induced ERK activation (Fig. 5a, b). Specific ERK depletion was active against everolimus-resistant gastric cancer cells (Fig. 5c, e). ERK promotes ubiquitination and degradation of Bim, a pro-apoptotic molecule member of Bcl-2 family proteins, resulting in resistance to chemotherapeutic agents in cancer cells [31, 32]. As a consequence of ERK inhibition, Bcl-2 family proteins were affected by trametinib as shown by the increased Bim and decreased Bcl-2 (Fig. 5a). We confirmed that trametinib overcame everolimus resistance by inhibiting ERK signaling and mediating Bcl-2 family proteins. This is supported by the previous work that the dual inhibition of mTOR and ERK is synergistic in other cancers, such as KRAS mutant lung cancer and cholangiocarcinoma [33, 34].
To our knowledge, we show for the first time that the paradoxical activation of ERK is one of the mechanisms leading to everolimus resistance in gastric cancer, and this can be reversed by the addition of trametinib. Our work serves as a proof-of-concept to suggest that the dual inhibition of ERK and mTOR is a better option than using single agent alone for gastric cancer.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65(1):5–29. https://doi.org/10.3322/caac.21254
Polkowski W, van Sandick JW, Offerhaus GJ, ten Kate FJ, Mulder J, Obertop H, van Lanschot JJ (1999) Prognostic value of Lauren classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol 6(3):290–297. https://doi.org/10.1007/s10434-999-0290-2
Ilson DH (2017) Advances in the treatment of gastric cancer. Curr Opin Gastroenterol 33(6):473–476. https://doi.org/10.1097/MOG.0000000000000395
Digklia A, Wagner AD (2016) Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol 22(8):2403–2414. https://doi.org/10.3748/wjg.v22.i8.2403
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, Group RS (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235. https://doi.org/10.1016/S1470-2045(14)70420-6
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22. https://doi.org/10.1016/j.ccr.2007.05.008
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. https://doi.org/10.1016/j.cell.2012.03.017
Saran U, Foti M, Dufour JF (2015) Cellular and molecular effects of the mTOR inhibitor everolimus. Clin Sci (Lond) 129(10):895–914. https://doi.org/10.1042/CS20150149
Oyama M, Sugiyama T, Nozawa M, Fujimoto K, Kishida T, Kimura G, Tokuda N, Hinotsu S, Shimozuma K, Akaza H, Ozono S (2017) Efficacy and safety of sequential use of everolimus in Japanese patients with advanced renal cell carcinoma after failure of first-line treatment with vascular endothelial growth factor receptor tyrosine kinase inhibitor: a multicenter phase II clinical trial. Jpn J Clin Oncol 47(6):551–559. https://doi.org/10.1093/jjco/hyw194
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, Group R-S (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372(9637):449–456. https://doi.org/10.1016/S0140-6736(08)61039-9
Mohamed A, Krajewski K, Cakar B, Ma CX (2013) Targeted therapy for breast cancer. Am J Pathol 183(4):1096–1112. https://doi.org/10.1016/j.ajpath.2013.07.005
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E (2013) Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 31(31):3935–3943. https://doi.org/10.1200/JCO.2012.48.3552
Shen YC, Li CP, Yen CJ, Hsu C, Lin YL, Lin ZZ, Chen LT, Su WC, Chao Y, Yeh KH, Cheng AL (2014) Phase II multicentered study of low-dose everolimus plus cisplatin and weekly 24-hour infusion of high-dose 5-fluorouracil and leucovorin as first-line treatment for patients with advanced gastric cancer. Oncology 87(2):104–113. https://doi.org/10.1159/000362671
Bailey ST, Zhou B, Damrauer JS, Kris-hnan B, Wilson HL, Smith AM, Li M, Yeh JJ, Kim WY (2014) mTOR inhibition induces compensatory, therapeutically targetable MEK activation in renal cell carcinoma. PLoS ONE 9(9):e104413. https://doi.org/10.1371/journal.pone.0104413
Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y (2019) Targeting mTOR for cancer therapy. J Hematol Oncol 12(1):71. https://doi.org/10.1186/s13045-019-0754-1
Butler DE, Marlein C, Walker HF, Frame FM, Mann VM, Simms MS, Davies BR, Collins AT, Maitland NJ (2017) Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 8(34):56698–56713. https://doi.org/10.18632/oncotarget.18082
Barranco SC, Townsend CM Jr, Casartelli C, Macik BG, Burger NL, Boerwinkle WR, Gourley WK (1983) Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res 43(4):1703–1709
Grimaldi AM, Simeone E, Festino L, Vanella V, Strudel M, Ascierto PA (2017) MEK inhibitors in the treatment of metastatic melanoma and solid tumors. Am J Clin Dermatol 18(6):745–754. https://doi.org/10.1007/s40257-017-0292-y
Balmanno K, Cook SJ (2009) Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ 16(3):368–377. https://doi.org/10.1038/cdd.2008.148
Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, Jia Z, Li Q, Yao JC, Xie K (2009) Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res 15(5):1821–1829. https://doi.org/10.1158/1078-0432.CCR-08-2138
Yuan R, Kay A, Berg WJ, Lebwohl D (2009) Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2:45. https://doi.org/10.1186/1756-8722-2-45
Lim T, Lee J, Lee DJ, Lee HY, Han B, Baek KK, Ahn HK, Lee SJ, Park SH, Park JO, Park YS, Lim HY, Kim KM, Kang WK (2011) Phase I trial of capecitabine plus everolimus (RAD001) in patients with previously treated metastatic gastric cancer. Cancer Chemother Pharmacol 68(1):255–262. https://doi.org/10.1007/s00280-011-1653-5
Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, Komatsu Y, Hamamoto Y, Ohno N, Fujita Y, Robson M, Ohtsu A (2010) Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 28(11):1904–1910. https://doi.org/10.1200/JCO.2009.26.2923
Taguchi F, Kodera Y, Katanasaka Y, Yanagihara K, Tamura T, Koizumi F (2011) Efficacy of RAD001 (everolimus) against advanced gastric cancer with peritoneal dissemination. Invest New Drugs 29(6):1198–1205. https://doi.org/10.1007/s10637-010-9464-9
Fukamachi H, Kim SK, Koh J, Lee HS, Sasaki Y, Yamashita K, Nishikawaji T, Shimada S, Akiyama Y, Byeon SJ, Bae DH, Okuno K, Nakagawa M, Tanioka T, Inokuchi M, Kawachi H, Tsuchiya K, Kojima K, Tokino T, Eishi Y, Kim YS, Kim WH, Yuasa Y, Tanaka S (2019) A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. J Exp Clin Cancer Res CR 38(1):127. https://doi.org/10.1186/s13046-019-1121-3
Salaroglio IC, Mungo E, Gazzano E, Kopecka J, Riganti C (2019) ERK is a pivotal player of chemo-immune-resistance in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms20102505
Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin TT, Chang CC, Jeng KS, Chang CF, Liu CH, Gao DY, Hsu FF, Duyverman AM, Kitahara S, Huang P, Dima S, Popescu I, Flaherty KT, Zhu AX, Bardeesy N, Jain RK, Benes CH, Duda DG (2017) Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep 7:44123. https://doi.org/10.1038/srep44123
Zeiser R, Andrlova H, Meiss F (2018) Trametinib (GSK1120212). Recent Results Cancer Res 211:91–100. https://doi.org/10.1007/978-3-319-91442-8_7
Hotz B, Keilholz U, Fusi A, Buhr HJ, Hotz HG (2012) In vitro and in vivo antitumor activity of cetuximab in human gastric cancer cell lines in relation to epidermal growth factor receptor (EGFR) expression and mutational phenotype. Gastric Cancer 15(3):252–264. https://doi.org/10.1007/s10120-011-0102-9
Tran TN, Brettingham-Moore K, Duong CP, Mitchell C, Clemons NJ, Phillips WA (2013) Molecular changes in the phosphatidylinositide 3-kinase (PI3K) pathway are common in gastric cancer. J Surg Oncol 108(2):113–120. https://doi.org/10.1002/jso.23357
Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E (2005) Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell 7(3):227–238. https://doi.org/10.1016/j.ccr.2005.02.008
Wang J, Zhou JY, Wu GS (2011) Bim protein degradation contributes to cisplatin resistance. J Biol Chem 286(25):22384–22392. https://doi.org/10.1074/jbc.M111.239566
Dogan Turacli I, Ozkan AC, Ekmekci A (2015) The comparison between dual inhibition of mTOR with MAPK and PI3K signaling pathways in KRAS mutant NSCLC cell lines. Tumour Biol 36(12):9339–9345. https://doi.org/10.1007/s13277-015-3671-0
Ewald F, Norz D, Grottke A, Hofmann BT, Nashan B, Jucker M (2014) Dual Inhibition of PI3K-AKT-mTOR- and RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK-inhibitors. Invest New Drugs 32(6):1144–1154. https://doi.org/10.1007/s10637-014-0149-7
Acknowledgements
This work was supported by a research grant provided by Health and Family Planning Commission of Hubei Province (Grant no. 2016-6).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Human participants and/or animals
All procedures involving animals were conducted according to the guidelines approved by the Institutional Animal Care and Use Committee of Hubei University of Arts and Science.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Yao, Y., Zhang, J. et al. MEK inhibition overcomes everolimus resistance in gastric cancer. Cancer Chemother Pharmacol 85, 1079–1087 (2020). https://doi.org/10.1007/s00280-020-04078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04078-0