Abstract
Gastric cancer is the third leading cause of cancer-related mortalities worldwide and mostly incurable. It remains an urgent need for novel strategies in the management of patients with advanced gastric cancer. Chimeric antigen receptor (CAR) T therapy has shown unprecedented clinical success in hematological malignancies and potential utility is going on various solid tumors like gastric cancer. In this study, a broad expression of NKG2D ligands was observed in gastric cancer cell lines, making them suitable targets for gastric cancer therapy. T cells were engineered with an NKG2D-based second-generation CAR and the resulting NKG2D-CAR-T cells showed significantly increased cytolytic activity against gastric cancer compared to untransduced T cells. In vivo, these cells can significantly suppressed the growth of established gastric cancer xenografts. Besides, cisplatin was shown to upregulate NKG2D ligand expression in gastric cancer cells and enhance the susceptibility to NKG2D-CAR-T-cell-mediated cytotoxicity. In conclusion, NKG2D-based CAR-T cells have potent in vivo and in vitro anti-tumor activities against gastric cancer and could be a new paradigm for patients with gastric cancer, either used alone or combined with chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is very common worldwide and ranks the third as the leading cause of cancer-related death globally [1, 2]. Despite the significant improvements in the past decades in screening and surveillance efforts for gastric cancer and advances in the multimodal approach based on gastrectomy, the overall patient survival rate of patients with advanced-stage disease is low, with a median overall survival uncommonly exceeding 10 months [2, 3]. Trastuzumab and ramucirumab (targeting HER2 and VEGFR2, respectively) remain as the only targeted therapies approved so far; however, an unsatisfactory survival benefit is gained and the development of drug resistance is arising [4, 5]. It is still urgent to identify better effective novel therapeutic strategies for patients with later stage gastric cancer.
Genetically engineered T-cell therapies provide a promising and clinically efficacious form of cell-based cancer immunotherapy [6, 7]. Particularly, the use of chimeric antigen receptors (CARs) that combine antigen specificity with signaling capability has rapidly advanced from the research filed of cancer treatment to the recent U.S. Food and Drug Administration (FDA) approvals of tisagenlecleucel (CTL019, Kymriah) and axicabtagene ciloleucel (Yescarta) for refractory B-cell leukemia and lymphoma, raising great interest by scientists and physicians searching for this platform approach to treat solid tumors, which occupy 85% of human tumor types [7, 8]. The impressive success of CAR-T therapies relies on the uniform and strong expression of CD19 on cells of B-cell lineage that is unique to hematologic malignancies. However, tumor-associated antigen targets exhibit heterogeneity with regard to intensity and distribution in solid tumors, raising questions concerning the ability to translate this platform of therapies to solid tumors [8, 9]. These challenges might be circumvented using ligand-targeted CAR-T therapies which involves harnessing the natural receptor/ligand interactions that are commonly dysregulated in transformed cell types [8]. Ligands for the natural killer group 2 member D (NKG2D) receptor are primarily expressed on many cancer types, but are absent in most non-transformed cells [10,11,12,13]. Several different variants of NKG2D-based CARs have been developed and its extensive efficacy against a range of tumors is documented [14,15,16,17].
Despite NKG2D-based CAR-T cells impressed us as an interesting approach for cancer treatment, their therapeutic potential has rarely been explored in treating gastric cancer. In this study, we find that NKG2D ligands, including MHC class chain-related (MICA/B) and UL16-binding protein families expression (ULBP1-3), are expressed in gastric cancer cell lines, making them suitable targets for gastric cancer therapy. We engineer human T cells with a second-generation NKG2D-CAR incorporating 4-1BB-CD3ζ signaling moieties and demonstrate that the NKG2D-CAR-T cells have potent in vivo and in vitro anti-tumor activities against gastric cancer. Cisplatin treatment is shown to induce the expressions of NKG2D ligands in gastric cancer cells and make them more susceptible to NKG2D-CAR-T-cell-mediated cytotoxicity. Together, our data reinforce the previous research and demonstrate this NKG2D-based CAR-T-cell therapies, either used alone or combined with chemotherapy, as a new legitimate option in the management patients with gastric cancer.
Materials and methods
Cell lines and cell culture
Human gastric cancer cell line MKN-28 was from the American-Type Culture Collection. Human gastric cancer cell lines SNU-1, SGC-7901, MKN-45, and several immortalized healthy cell lines, including HMEC-1, GES-1, and THLE-3 were obtained from Shanghai cell bank of Chinese Academy of Sciences. Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, USA) with 10% fetal bovine serum (FBS) (GIBCO, USA) was used to maintain all cell lines. All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. Under the same experimental protocol, their cell marker profile was periodically analyzed by flow cytometry to ensure that no changes had occurred.
Construction of NKG2D-based CAR
The extracellular portion of human NKG2D (amino acids 82–216) and 4-1BB-CD3ζ expression cassette was designed and synthesized by the GeneWiz Biotechnology Company (Soochow, China). Together, the second-generation NKG2D-based CAR construct consisted of the extracellular portion of NKG2D (nucleotides 82–216, GenBank NM 007360.3), linking via a CD8α hinge-transmembrane domains (nucleotides 412–546, GenBank NM 001768.6) to the intracellular signaling domains of 4-1BB (nucleotides 640–765, GenBank NM 006139.3) and CD3ζ molecule (nucleotides 154–492, GenBank NM 198253.2) followed by enhanced green fluorescent protein (eGFP) through a ribosomal skipping sequence (F2A).
Lentivirus production and concentration
The complete CAR sequence was cloned into the SalI, XhoI restriction site of the lentiviral PCDH-CMV vector backbone (Invabio, Shanghai, China), yielding PCDH-CMV-NKG2D-4-1BBζ-F2A-eGFP. The PCDH-CMV-F2A-eGFP presented as a control vector. Lentiviral particles containing the NKG2D-CAR or mock control were produced by transfection of 95% confluent 293T cells with the aforementioned vectors, together with the packaging constructs using calcium phosphate transfection reagent (Promega, USA). Seventy-two hour post-transfection, the conditioned medium was harvested and filtered through a 0.45 µM filter unit (Millipore, USA). High-titer lentiviral particles were concentrated 30-fold by ultracentrifugation (Beckman, USA) for 2 h at the speed of 28,0000 rpm. The pallet was resuspended with 1 × PBS.
Isolation, activation, transduction, and expansion of NKG2D-based CAR-T cells
Peripheral blood samples were obtained from healthy adults in Shaoxing blood center and peripheral blood mononuclear cells (PBMCs) were separated by Ficoll density gradient centrifugation. Briefly, PBMCs were stimulated with magnetic beads coated with anti-CD3/anti-CD28 antibodies (Invitrogen, USA) at a 2:1 cell-to-bead ratio for 24 h and cells were then transduced with the lentiviral particles. Transduction was performed using 5 × 106 PBMCs in 24-well plates mixed with the corresponding lentiviral particles at a multiplicity of infection (MOI) of 5 in a total volume of 500 µL containing 8 µg/ml polybrene (Sigma-Aldrich, USA). The transduced T cells were cultured at a concentration of 5 × 105 cells/mL and recombinant human interkin-2 (rhIL-2, 500 IU/mL) (Huaxin HighTech, China) were added every other day. Genetically modified T cells were used for functional assays when T cells appeared to become quiescent, as determined by decreased lymphocyte volume and growth kinetics.
Flow cytometry analyses
Surface expression of NKG2D-CAR expression on transduced T cells was evaluated by staining with goat anti-human PE-anti-NKG2D antibody (clone 1D11, BD Biosciences, USA). The expression of T-cell phenotypes was determined 14 days of cell expansion using mouse anti-human antibodies to PE-CD4 (clone RM4-5), PE-CD8 (clone 3B5), FITC-CD62L (LT-TD180), and PE-CCR7 (clone 4B12) from ebiosciences. To evaluate expression of NKG2D ligands on gastric cancer cells, the cells were stained with PE-anti-MICA/B (clone 6D4), PE-anti-ULBP1 (clone 170818), PE-anti-ULBP2 (clone 16590), and APC-anti-ULBP3 (clone 2F9) from R&D Systems. The results were confirmed by incubating gastric cancer cell lines with human recombinant NKG2D/Fc chimera (R&D Systems, USA), followed by PE-conjugated goat anti-human IgGFc (R&D Systems, USA). Degranulation ability was assessed by flow cytometric analysis of CD107a expression. NKG2D-CAR- or mock-transduced T cells were co-cultured with medium, MKN-28 in the absence or the presence of an anti-NKG2D blocking antibody (Clone#552866, BD Biosciences, USA), and an anti-NKG2D agonistic antibody (Clone1D11, eBiosciences, USA) coated at plate for 6 h. Anti-human CD107a antibody conjugated to phycoerythrin (BD Biosciences, USA) was added at the beginning of the cultures and 1 h later GolgiStop (BD Biosciences, USA) was added. Flow cytometry was performed with a BD FACSCanto II flow cytometer (BD Biosciences, USA) and flow cytometric data were analyzed with the FlowJo version 7.2.5 software (Tree Star, USA).
In vitro cytotoxicity assays
We measured the in vitro cytotoxic activity of genetically modified T cells using Lactate dehydrogenase (LDH)-releasing assays with Non-radioactive Cytotoxicity Assay Kit (Promega, USA) according to the manufactures’ instructions. Briefly, NKG2D-CAR- or mock-transduced T cells were co-cultured for 6 h at effector:target (E:T) ratio of 10:1, 5:1, and 2.5:1 in a final volume of 100 µL in 96-well plates at 37 °C in a humidified atmosphere containing 5% CO2. For blocking assays, NKG2D-CAR-T cells were exposed to a blocking anti-NKG2D mAb or an isotype- matched control antibody for 45 min, followed by co-incubating with target cells as above. In some experiments, gastric cancer cells were treated for 48 h with cisplatin (Tasly Diyi Pharmaceutical, China), and then processed for the NKG2D-CAR-T-cell-mediated cytotoxicity. Following this, the supernatant was obtained by centrifugation for 5 min at 200×g and 50 µL of supernatant from each well were transferred into a microtiter 96-well plate. LDH solution (50 µL) was added to the supernatant, and then, the mixture was measured at 490 nm using the microtiter plate reader. The spontaneous release of effector or target cells and maximum release of target cells were also measured. The percentage of cell lysis was calculated as follows: Cell lysis (%) = 100 × (experimental release − target spontaneous release − effector spontaneous release)/(maximal release − spontaneous release).
CCK-8 assay
Cells were prepared at a density of 5 × 104/mL after trypsinization and inoculated into a 96-well culture plate. The liquid was discarded after 12 h culturing, while an increasing low dose of cisplatin (0.5 µM, 1 µM, and 2 µM) was added. After cisplatin treatment for 48 h, attached cells were incubated with CCK-8 (10×) for 2 h and then followed by measuring the absorbance of each well at 450 nm. Each data represent at least 3 triplicates. The inhibition rates (%) were calculated as the formula:
Cytokine release assay
Cytokine release assays were performed in triplicate in 96-well V-bottom plates, using NKG2D-CAR- or mock-transduced T cells co-culturing with different gastric cancer cells at an E:T ratio of 5:1 at 37 °C in a humidified atmosphere containing 5% CO2. After 18 h, cell-free supernatants were collected for interferon-γ (IFN-γ), tumor necrosis factor (TNF-α) and granulocyte–macrophage colony stimulating factor (GM-CSF) production by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, USA) in accordance with the manufacturer’s protocols.
Murine models
All animal work was authorized by the Medical Experimental Animal Care Commission of Shaoxing People’s Hospital and the methods were carried out in accordance with the applicable guidelines. Female NOD.Cg-Prkdcscid IL2rgtm1WjI/SzJ (NOD/scid IL2RG-null, NSG) mice age 6–8 weeks were purchased from the Model Animal Research Center of Nanjing University, housed and treated in microisolator cages under specific pathogen free conditions in the facility of Shaoxing People’s Hospital. Subcutaneous MKN-28 xenografts were established by inoculating NSG mice with 5 × 106 cells at the right or left flank. One week after MKN-28 cell implantation, when the tumor burden reached approximately 100–200mm3, mice were assigned to receive 5 × 106 NKG2D-CAR-T cells, 5 × 106 mock-transduced T cells or PBS as the untreated control via caudal vein injections (n = 6). Tumor growth was measured twice a week using Vernier calipers and tumor volumes were calculated as the formula: V = length × (width)2 × 0.5. When animal studies were terminated, all mice were sacrificed and tumor tissues were surgically excised, fixed with formalin, embedded in paraffin, and serially sectioned at a 4-µm thickness for future use.
T-cell infiltration in tumor
We prepared several mice bearing subcutaneous MKN-28 xenografts. When tumor volume was approximately 100 mm3, NKG2D-CAR- or mock-transduced T cells were injected into the lateral tail vein of the tumor-bearing mice (1 × 107 cells). A second injection was carried out 1 week later. Fourteen days after the first injection, mice were euthanized, tumors were resected, and single-cell suspensions were prepared enzymatically by incubating the specimens for 45 min in RPMI-1640 medium containing 0.5-mg/mL pronase, 1.5-mg/mL collagenase D, and 500-µg/mL DNase I (Sigma, USA). After centrifugation at 1000 rpm for 5 min and filtration through a 40-µm nylon cell strainer, the cells were stained with PE-anti-NKG2D antibody (clone 1D11, BD Biosciences, USA) for 45 min, washed and underwent flow cytometry.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using the TRIzol reagent (Invitrogen, USA) and was reverse transcribed into cDNA using PrimeScript RT Reagent Kit (TaKaRa, China). Real-time PCR was performed with 20 ng of RNA on an SYBR Green real-time PCR detection system to determine the mRNA expression level of a gene of interest according to the manufacturer’s instructions. Primers purchased from Genewiz (Soochow, China) were shown in Supplementary Table S1. Expression levels were calculated by the 2−ΔΔct method compared to the expression of glyceraldehydes phosphate dehydrogenase (GAPDH).
Immunohistochemistry (IHC) analysis
Standard IHC staining procedures were performed on specified sections for analysis of T-cell infiltration with mouse anti-human NKG2D antibody (1:500 dilution, Abcam, USA) and for detection of proliferation with mouse anti-human PCNA antibody (1:500 dilution, Abcam, USA). The primary antibody was incubated at 4 °C overnight. Detection of primary antibody was achieved using horseradish peroxidase (HRP) conjugated secondary goat anti-mouse/rabbit immunoglobulin G (IgG) (ZSQJB, Beijing, China). DAB chromogen eventually showed color (Tiangen Biotech, China). Nuclei were counterstained with hematoxylin. Appropriate negative controls were prepared in which the primary antibody was replaced with 1 × PBS. PCNA protein appeared as cytoplasmic brown–yellow staining and NKG2D staining was mainly distributed in the cell membrane. A scoring system taking into account the staining intensity and percentage of positive staining tumor cells was applied to measure PCNA expression. The percentage of positive stained tumor cells per section was scored into five groups: < 5.0% = 0; 5.1–25.0% = 1; 25.1–50.0% = 2; 50.1–75.0% = 3; and > 75.0% = 4. The intensity was assessed as: no staining = 0; weak staining = 1; moderate staining = 2; and strong staining = 3. The IHC score was calculated from “area × intensity” and categorized into four group: score 0–2 (−); score 3–5 (+); score 6–8 (++); and score 9–12 (+++). The numbers of infiltrating NKG2D-positive T cells per ten high-power fields in tumors collected from mice treated with either mock-transduced T and NKG2D-CAR-T cells were countered under high-power microscope.
Statistics
Data are presented as the means ± standard error of the mean (SEM) or standard deviation (SD). Difference between groups was analyzed using two-tailed Student’s t tests or one-way ANOVA with Bonferroni post-tests. *p < 0.05, **p < 0.01, and ***p < 0.001 were considered statistically significant. All statistical analyses were performed using Graphpad Prism 5.0.
Results
Expression of NKG2D ligands in human gastric cancer cell lines
To initially identify the expression profile of NKG2D ligands in gastric cancer, a panel of established gastric cancer cell lines including MKN-28, SNU-1, MKN-45 and SGC-7901 were stained for the expression of various NKG2D ligands, including MICA/B, ULBP1, ULBP2 and ULBP3. Data from flow cytometric analyses demonstrated that MKN-28 expressed high levels of MICA/B and ULBP2 but did not express detectable ULBP1 and ULBP3. MKN-28 cells did not express MICA/B and ULBP2 but have moderate expression levels of ULBP1 and ULBP3. SNU-1 cells expressed ULBP2 but did not express the others. SGC-7901 cells marginally expressed ULBP1, ULBP2 and ULBP3 but did not express MICA/B (Fig. 1). Furthermore, we applied a human recombinant NKG2D/Ig Fc reagent to evaluate the collective expression of NKG2D ligands. These cell lines indeed showed labeling with NKG2D/Ig Fc, corresponding to the respective staining of NKG2D ligands (Fig. 1). Though the expression NKG2D ligands were mostly scattered, we have observed a broad expression of NKG2D ligands in gastric cancer, indicating the potentially NKG2D/NKG2D ligands interactions may be potentially exploited to treat human gastric cancer.
NKG2D ligands expression in human gastric cancer cell lines. Flow cytometric analyses of expression of NKG2D ligands on the surface of gastric cancer cell lines. Human gastric cancer cell lines were stained with specific antibodies recognizing MICA/B, ULBP-1, ULBP-2, ULBP-3, and NKG2D-Fc. The same cells stained with isotype antibody were used for gating (black). Data shown are representatives of experiments with similar results
Generation of NKG2D-based CAR-T cells by lentiviral vector transduction
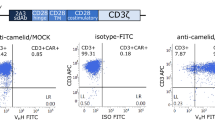
The second-generation NKG2D-CAR construct consisted of the extracellular portion of NKG2D, linking via a CD8α hinge-transmembrane domains to the intracellular signaling domains of 4-1BB and CD3ζ molecule (nucleotides 154–492, GenBank NM 198253.2) followed by eGFP expression cleaved via a F2A skipping sequence (Fig. 2a). We generated human NKG2D-CAR- or mock-transduced T cells by lentiviral transduction of PBMCs derived from healthy donors. NKG2D-CAR-T cells were identified double-positive staining of NKG2D and GFP. We performed the flow cytometry 10–14 days after transfection. NKG2D cell surface expression levels in NKG2D-CAR-T cells were significantly higher than mock-transduced T cells, indicating that NKG2D-based CAR was successfully introduced in NKG2D-CAR-T cells (Fig. 2b). To characterize the phenotypes of the T-cell products, the cells were harvested for flow cytometric detection of CD4, CD8, CD62L and CCR7 by day 14 after transfection. Majority of the T cells were CD8-positive after a period of expansion in vitro, which occurred independently of the gene transduction of T cells (Figure S1). This was beneficial as that a high CD8/CD4 ratio has been shown to be an effective indicator for better outcome of adoptive T-cell immunotherapy against cancers [18]. Majority T cells were CD62L-positive and no significant variation of this marker was observed between two groups, indicating a differentiated phenotype of T cells irrespective of gene transfection (Figure S1). Like mock-transduced T cells, NKG2D-CAR-T cells contained 8.5 ± 3.2% cells expressed CCR7, indicating that the candidate T cells had a certain homing capacity (Figure S1). Altogether, CAR modification did not change the phenotypic characterization of T cells.
Construction and expression of NKG2D-CAR in transduced T cells. a Schematic representation of NKG2D-based CAR and the lentiviral vector. The construct consisted of the extracellular portion of NKG2D, linking via a CD8α hinge-transmembrane domains to the intracellular signaling domains of 4-1BB and CD3ζ molecule followed by eGFP expression cleaved via a F2A skipping sequence. eGFP was included for the detection of genetically modified T cells. b Flow cytometric analysis of NKG2D-CAR expression on the surface of NKG2D-CAR-T cells as evidenced by the co-expression of NKG2D receptor and eGFP. Gating was based on the same cells stained with isotype-matched antibody. Data shown are representatives of experiments with similar results
Enhanced cytototoxicity of NKG2D-CAR-T cells against gastric cancer cell lines
To determine whether gastric cancer cell lines were susceptible to NKG2D-CAR-T-cell-mediated lysis in vitro, we performed cytotoxicity assays using engineered T cells as effector cells and different gastric cancer cells as target cells. Compared to mock-transduced T cells, NKG2D-CAR-T cells displayed significantly higher specific cytolytic activity against several gastric cancer cell lines in an E:T-dependent manner (Fig. 3a). To confirm whether the increase in cytotoxicity triggered by transduction of the NKG2D-based CAR was directly dependent on NKG2D signaling, we used an anti-NKG2D blocking antibody in the co-culture system. In the blocking experiment, the gains in cell lysis triggered by NKG2D-CAR-T cells against MKN-28 was markedly diminished in the context of the blocking antibody but not the irrelevant isotype antibody control, confirming the NKG2D dependency of NKG2D-CAR-T cells (Fig. 3b). It was reported that NKG2D ligands exist as highly suppressive soluble forms in the blood serum of patients with metastatic diseases [19, 20]. To investigate whether soluble NKG2D ligands can hinder the anti-tumor activity of NKG2D-CAR-T cells, their cytotoxicities against MKN-28 cells were determined in the presence or absence of soluble NKG2D ligands. As shown in Fig. 3c and in consistent with a previous study, NKG2D-based CAR inhibition does not occur under physiological concentrations of soluble recombinant ligands [20].
Enhanced cytototoxicity of NKG2D-CAR-T cells against gastric cancer cell lines. a Cytotoxic activity of NKG2D-CAR- or mock-transduced T cells against gastric cancer cell lines. The effector cells were co-cultured for 6 h with target cells (1–2 × 104) at E:T ratio of 10:1, 5:1 and 2.5:1 in a total volume of 100 µL, respectively. b Cytotoxicity of NKG2D-CAR-T cells against MKN-28 in the presence of an anti-NKG2D blocking antibody or irrelevant control at E:T ratio of 10:1, 5:1 and 2.5:1 for 18 h. c Cytotoxicity of NKG2D-CAR-T cells against MKN-28 in the presence of mammalian cell-expressed soluble MICA and ULBP-2 at an E:T ratio of 5:1 for 18 h. ns not significant. Cytotoxicity was determined by LDH release assays. Data reflect the mean ± SEM of three separate experiments. *p < 0.05, **p < 0.01 and ***p < 0.001, compared with mock-transduced T cells at the same E:T ratios or concentrations
Enhanced CD107a and cytokine production of NKG2D-CAR-T cells
We further analyzed the cell surface expression of the lysosomal-associated membrane protein-1 (LAMP-1 or CD107a), which has been shown to be a sensitive marker for immune cell functional activity [21]. As shown in Fig. 4a, significantly higher lysosomal granule exocytosis, as measured by CD107a expression, was observed in NKG2D-CAR-T cells compared to that in mock-transduced T cells in response to MKN-28 or an anti-NKG2D agonistic antibody, and there was no significant difference in response to medium or MKN-28 in the context of an anti-NKG2D blocking antibody, confirming NKG2D-dependent functional superiority of NKG2D-CAR-T cells. Chronic NKG2D engagement may result in suppression of NK cell function. Indeed, mock-transduced T cells failed to degranulate after 18 h of chronic stimulation, whereas a substantial of NKG2D-CAR-T cells were still CD107a positive after prolonged stimulation (Fig. 4b). Cytokines are important effector molecules of CAR-T-cell function, we assessed whether the this NKG2D-CAR-T-cell-mediated killing accompanied with an increased cytokine secretion. The data showed that much higher levels of IFN-γ, and moderate higher levels of TNF-α and GM-CSF were produced by NKG2D-CAR-T cells compared to mock-transduced T cells when co-culturing with MKN-28 cells (Fig. 4c).
Enhanced CD107a and cytokine production of NKG2D-CAR-T cells. a Representative flow cytometric dot plots illustrating CD107a expression on NKG2D-CAR- or mock-transduced T cells after 6 h of co-culture with medium, MKN-28, MKN-28 in the presence of an anti-NKG2D blocking antibody or an anti-NKG2D agonistic antibody. The same cells stained with isotype-matched controls were used for gating. b Representative flow cytometric dot plots illustrating CD107a expression on NKG2D-CAR- or mock-transduced T cells after long-term co-culturing with an anti-NKG2D agonistic antibody. c IFN-γ, TNF-α and GM-CSF production by NKG2D-CAR-T cells or mock-transduced T cells when co-cultured with the indicated cells at E:T ratio of 5:1 for 18 h. The analyses were performed using Students’ t tests. ***p < 0.001, compared with that of mock-transduced T cells
Therapeutic efficacy of NKG2D-CAR-T cells against gastric cancer xenografts
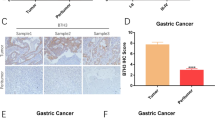
We next assessed whether NKG2D-CAR-T cells similarly suppressed the growth of established gastric cancer xenografts. NSG mice bearing subcutaneous MKN-28 xenografts were established. When tumor volumes were approximately 100–200 mm3, NKG2D-CAR-T or mock-transduced T cells or the injection medium PBS were administrated via the tail vein. Figure 5a shows that adoptive transfer of NKG2D-CAR-T cells markedly inhibited the growth of the MKN-28, while no significant tumor growth delay was observed in the mice receiving mock-transduced T cells or PBS. We next examined whether NKG2D-CAR-T cells can accumulate in the tumor site. Fourteen days after T-cell injections, tumor from mice was analyzed by flow cytometry for cells expressing eGFP and NKG2D. The results revealed that only T cells engineered with NKG2D-CAR, but not mock were detectable in appreciable numbers in the sections of tumors (Fig. 5b). The results of IHC assays confirmed that NKG2D-CAR-T cells accumulated much higher in residual tumors, as evidenced by significantly more specific staining was observed in the tumor sections from mice treated with NKG2D-CAR-T cells compared with mice that treated with mock-transduced T cells or PBS (Fig. 5c, d). We further immunostained for detection of proliferation with mouse anti-human PCNA antibody. A decrease in PCNA staining was observed in the MKN-28 tumors harvested from mice treated with NKG2D-CAR-T cells rather than mice treated with mock-transduced T cells or PBS (Fig. 5c, e). These results demonstrated that NKG2D-CAR-T cells can accumulate in tumors and exhibit anti-tumor efficacy via proliferation inhibition in tumor cells.
Therapeutic efficacy of NKG2D-CAR-T cells against gastric cancer xenografts. a Growth curve of MKN-28 subcutaneous xenografts treated with the indicated T cells (5 × 106/mouse) or PBS (n = 6). Treatments were administrated when tumor volumes reached approximately 100–200 mm3. Black arrows represented the days on which the indicated treatments were delivered. b Fourteen days after T-cell injections, tumor from mice was analyzed by flow cytometry for cells expressing NKG2D and eGFP. c Representative tumor sections stained with anti-NKG2D and anti-PCNA antibodies were shown. The specimens were harvested from MKN-28 xenografts sacrificed after study terminated. Nuclei are stained with hematoxylin. Magnification: 200. d, e The quantitative data of c. Data are presented as the mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001, compared with mice treated with mock-transduced T cells
Interaction between NKG2D-CAR-expressing CAR-T cells and cisplatin
Future cancer treatment should rely on combination therapies and may implement novel immunotherapeutic strategies such as CAR-T-cell therapy. NKG2D–NKG2DL pathway plays an important role in controlling tumor progression and this interaction can be enhanced by various chemotherapeutic agents or irradiation treatment. We were interested to see whether the combination of NKG2D-CAR-T therapy with cisplatin, a chemotherapy agent commonly used to treat advanced gastric cancer may result in synergistic activity. Gastric cancer cell lines were treated with cisplatin at low concentrations (0.5 and 2 µM) for 48 h and subsequently evaluated by CCK-8 assays. Little effect was found on gastric cancer cell viability at these low concentrations (Figure S2). Interestingly, our results revealed that low dosages of cisplatin can enhance the susceptibility of SGC-7901 and SNU-1 cells to NKG2D-CAR-T-cell-mediated cytolytic activity (Fig. 6a). Moreover, higher IFN-γ and TNF-α release were observed in conditioned media collected from NKG2D-CAR-T cells co-cultured with tumor cells that were pretreated with cisplatin (Fig. 6b). These data demonstrated that low dose of cisplatin could sensitize gastric cancer cells to NKG2D-CAR-T-cell-mediated lytic activity, thus further improving the therapeutic efficacy to combat against gastric cancer.
Cisplatin sensitize gastric cancer cell lines to NKG2D-CAR-T-cell-mediated immunotherapy efficacy. a SGC-7901 and SUN-1 were pretreated with cisplatin at increasing low dosages of cisplatin (0.5 and 2 µM) for 48 h and processed for NKG2D-CAR- or mock-transduced T-cell cytotoxicity testing in an E:T ratio of 5:1 for 18 h. b Conditioned media from a were collected to test IFN-γ and TNF-α release from NKG2D-CAR- or mock-transduced T cells. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, compared with gastric cancer cells not pretreated with cisplatin
Cisplatin enhanced NKG2D-CAR-T-cell-based immunotherapy efficacy via increasing the expression of NKG2D ligands in gastric cancer
As it has been demonstrated that cisplatin pretreatment could sensitize gastric cancer cell lines to NKG2D-CAR-T-cell-mediated cytotoxicity and the expression of NKG2D ligands on tumor cells is necessary for recognition by NKG2D-CAR-T cells, we next investigated whether cisplatin induce the synergistic killing via increasing the expression of NKG2D ligands. The effect of cisplatin on the expression of various NKG2D ligand proteins was determined in SGC-7901 and SNU-1 cells. We found that compared to the vehicle control group, low dose of cisplatin treatment could significantly increase mRNA transcription and surface expression of MICA/B genes in MKN-28 human gastric cancer cell line. Besides, MICA/B and ULBP2 gene expressions were significantly induced in SUN-1 cells (Fig. 7a and Figure S3A). Nevertheless, cisplatin did not elicit up-regulation of NKG2D ligands in PBMC and several immortalized healthy cell lines, including HMEC-1, GES-1 and THLE-3 (Figure S3B). To determine if NKG2D signaling was involved in the increased susceptibility of cancer cells to NKG2D-CAR-T-cell-mediated cytotoxicity after cisplatin, we applied a blocking NKG2D mAb in the co-culture system. Interestingly, when blocking anti-NKG2D antibody was added, the increased cytotoxicity of NKG2D-CAR-T cells against cisplatin treated cancer cells was abolished, suggesting that cisplatin should make gastric cancer cells more vulnerable to NKG2D-CAR-T cells via induction of NKG2D ligands on tumor cells (Fig. 7b).
Cisplatin induced up-regulation of NKG2D ligands and enhanced NKG2D-CAR-T-cell immunotherapy efficacy against gastric cancer. a Surface expression of NKG2D ligands in SGC-7901 and SUN-1 cells after cisplatin treatment (0.5 and 2 µM) for 48 h. b For blocking assays, NKG2D-CAR- or mock-transduced T cells were preincubated with anti-NKG2D antibody or isotype control. The cell-mediated cytotoxicity was determined against MKN-45 and SUN-1 cells pretreated with cisplatin or not. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, compared with gastric cancer cells not pretreated with cisplatin
Discussion
In this study, we demonstrated that NKG2D-based CAR-T cells have potent in vivo and in vitro anti-tumor activities, which might be applicable to patients with NKG2D ligand-positive gastric cancer. Besides, we showed that cisplatin can induce up-regulation of NKG2D ligands expression in gastric cancer cell lines, rendering these malignant cells more sensitive to NKG2D-based CAR-T-cell-mediated killing. These results justify clinical translation of this NKG2D-based CAR-T therapies, either used alone or combined with chemotherapy, as a novel therapeutic option for patients with gastric cancer.
The standard CAR-T therapy targeting CD19 antigen has consistently produce positive results in patients with hematologic malignancies, underscoring its great potential in anti-tumor therapy [6, 8]. However, beyond CD19 CAR T-cell therapy, standard scFv-based CARs have failed to elicit significant clinical responses in solid tumors, questioning the generic applicability of the approach [22,23,24,25,26]. Tumor heterogeneity, characterized by varying levels of expression intensity and distribution of antigen-positive cells, present a substantial challenge to increasing clinical responses mediated by CAR-T-cell therapy [9]. An strategy to deal with tumor heterogeneity was to exploit natural receptor/ligand interactions in CAR designs [8, 27, 28]. This expands the potential range of the CAR and reduces the likelihood of tumor resistance due to outgrowth of target antigen negative cancer cells or tumor relapse due to antigen escape variants. Solid tumors often create an immunosuppressive microenvironment that poses a formidable challenge to CAR-T-cell therapy [29,30,31]. However, it has been well appreciated that NKG2D, one of the most important natural activating receptors for cancer immunosurveillance, can also target immunosuppressive or other cells in the tumor microenvironment, rendering NKG2D-based CAR-T cells with the ability to modulate the host microenvironment to promote anti-tumor immunity [32, 33]. Though somewhat unilaterally, these studies are valuable for demonstrating that immunotherapies based on NKG2D-based CAR-T cells might be promising for the future development against cancers.
NKG2D has multiple ligands including MICA/B and ULBP1-6, which are preferentially expressed after cellular stress, infection and DNA damage [34]. Here we validated the expression of NKG2D ligands in gastric cancer at the protein level, demonstrating that NKG2D ligands were widely expressed among human gastric cancer cell lines. In the present study, we observed a broad expression of NKG2D ligands in gastric cancer, indicating the potentially NKG2D/NKG2D ligands interactions may be potentially exploited to treat human gastric cancer. We engineered human T cells with a NKG2D-based second-generation CAR and the modified CAR-T cells can recognize and kill NKG2D ligand-positive gastric cancer cells. Gains in cytotoxicity of NKG2D-CAR-T cells were dependent on NKG2D-mediated signaling, as this response could be blocked by an antagonist an-NKG2D antibody and it was dependent on NKG2D ligand expression on the target cells. Chronic NKG2D engagement may result in suppression of NK cell function [35]. Indeed, mock-transduced T cells failed to degranulate after 18 h of chronic stimulation, whereas a substantial of NKG2D-CAR-transduced T cells were still CD107a positive after prolonged stimulation. Additionally, NKG2D-based CAR inhibition did not occur under physiological concentrations of soluble recombinant ligands.
Upon antigenic stimulation, T cells can produce large amounts of cytokines. Cytokines appear to be a key mechanism used by CAR-T cells to change the tumor microenvironment and to induce host anti-tumor immunity. Here, we found that NKG2D-CAR-bearing T cells produced high levels of IFN-γ and moderate levels of TNF-α and GM-CSF, which were similar to what has been observed from activated human tumor-specific cytotoxic tumor lymphocytes. IFN-γ can induce activation of bystander-immune cells and enhance endogenous anti-tumor immunity [36, 37]. GM-CSF alone or in combination with TNF-α plays important roles in generation of mature dendritic cells as well as in tumor antigen presentation [38]. In this study, the gains of NKG2D-mediated anti-tumor activity were obvious in established gastric cancer xenografts, where NKG2D-CAR-T cells produced marked tumor reductions, while mock-transduced T cells were less effective. Obvious infiltration of T cells were found in the tumor specimens receiving CAR-T treatments, suggesting that NKG2D-CAR-T cells can traffic to tumors and induce anti-tumor responses.
Although NKG2D-based CARs have the potential to recognize approximately 90% of human tumor type, there are many gastric cancer patients with moderate or low expression levels of NKG2D ligands. Besides, the down-regulation of NKG2D ligands on malignant cells was a general mechanism to evade NKG2D-mediated immune response [39, 40]. One possible way to improve tumor targeting through NKG2D would be to increase the expression of NKG2D ligands on malignant cells or at the tumor site. Here we showed that cisplatin treatment can up-regulate the expression of NKG2D ligands on tumor cells and thus sensitize gastric cancer cell lines to NKG2D-based CAR-T-cell-mediated killing, which potentially expand the therapeutic window of this platform of CAR technology. It is possible that treatment with cisplatin could be used to increase ligand expression on tumor cells; however, the induction of ligands on normal cells must be avoided to prevent unwanted toxicity. We treated non-transformed cells with cisplatin, finding that exposure to cisplatin did not result in enhanced expression of NKG2D ligands on PBMCs and several immortalized healthy cell lines, excluding the likelihood of enlarging toxicity. The exact mechanism underlying NKG2D ligand expression has not been elucidated in this study, it is possible that ciaplatin might trigger DNA damage and increase expressions of NKG2D ligands.
Several limitations should be considered while interpreting some results. First, the DNAX-activating protein (DAP10) was reported to promote and stabilize NKG2D expression, whereas we did not incorporate the molecule into the NKG2D-based CAR construct due to that we have engineered the NKG2D binding domain to a standard second-generation CAR configuration [41, 42]. It might be interesting to explore whether DAP10 would enhance CAR expression in the present situation. Second, we engineered human NKG2D-based CAR construct that does not recognize murine NKG2D ligands and potential toxicity associated with NKG2D-based CAR-T cells could not be evaluated in the present mouse models. Last but not least, unlike conventional antigen-targeting strategies, NKG2D can target tumor cells, immunosuppressive cells and other cells in the microenvironment, the immunocompromised mice employed in this study, however, lack a functioning immunosuppressive milieu to better show CAR effectiveness.
In conclusion, our results highlights the potential for the development of NKG2D-based CAR-T cells for cancer immunotherapy, either used alone or combined with cisplatin, that represent a novel therapeutic option for patients with gastric cancer.
References
Torre LA et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Van Cutsem E et al (2016) Gastric cancer. Lancet 388(10060):2654–2664
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Javle M, Smyth EC, Chau I (2014) Ramucirumab: successfully targeting angiogenesis in gastric cancer. Clin Cancer Res 20(23):5875–5881
Fujita T (2010) Trastuzumab for gastric cancer treatment. Lancet 376(9754):1735 (author reply 1735–6)
Kochenderfer JN, Rosenberg SA (2013) Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 10(5):267–276
June CH et al (2018) CAR T cell immunotherapy for human cancer. Science 359(6382):1361–1365
Chen N et al (2018) Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr Opin Immunol 51:103–110
Li J et al (2018) Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 11(1):22
McGilvray RW et al (2009) NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res 15(22):6993–7002
Pende D et al (2002) Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res 62(21):6178–6186
Friese MA et al (2003) MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res 63(24):8996–9006
Salih HR et al (2003) Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 102(4):1389–1396
Barber A, Meehan KR, Sentman CL (2011) Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther 18(5):509–516
Barber A, Sentman CL (2009) Chimeric NKG2D T cells require both T cell- and host-derived cytokine secretion and perforin expression to increase tumor antigen presentation and systemic immunity. J Immunol 183(4):2365–2372
Zhang T, Lemoi BA, Sentman CL (2005) Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood 106(5):1544–1551
Lehner M et al (2012) Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One 7(2):e31210
Turtle CJ et al (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Investig 126(6):2123–2138
Diefenbach A, Raulet DH (2002) The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol Rev 188:9–21
Zhang T, Barber A, Sentman CL (2006) Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res 66(11):5927–5933
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1–2):15–22
Gilham DE et al (2012) CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med 18(7):377–384
Zhang C et al, Phase I, Escalating-Dose (2017) Trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol Ther 25(5):1248–1258
Junghans RP et al (2016) Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76(14):1257–1270
Mason NJ et al (2016) Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res 22(17):4380–4390
You F et al (2016) Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci 59(4):386–397
Spear P et al (2013) NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol 91(6):435–440
Eisenberg V et al (2017) Targeting multiple tumors using T-cells engineered to express a natural cytotoxicity receptor 2-based chimeric receptor. Front Immunol 8:1212
Beatty GL, Moon EK (2014) Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 3(11):e970027
Mohammed S et al (2017) Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol Ther 25(1):249–258
Scarfo I, Maus MV (2017) Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer 5:28
Barber A, Rynda A, Sentman CL (2009) Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol 183(11):6939–6947
Zhang T, Sentman CL (2013) Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T cells. J Immunol 190(5):2455–2463
Dhar P, Wu JD (2018) NKG2D and its ligands in cancer. Curr Opin Immunol 51:55–61
Koch C et al (2017) Chronic NKG2D engagement in vivo differentially impacts NK cell responsiveness by activating NK receptors. Front Immunol 8:1466
Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13(2):95–109
Schroder K et al (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75(2):163–189
Zhan Y et al (2012) The inflammatory cytokine, GM-CSF, alters the developmental outcome of murine dendritic cells. Eur J Immunol 42(11):2889–2900
Song H et al (2006) Soluble ULBP suppresses natural killer cell activity via down-regulating NKG2D expression. Cell Immunol 239(1):22–30
Kamimura H et al (2012) Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol 56(2):381–388
Wu J et al (1999) An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285(5428):730–732
Garrity D et al (2005) The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA 102(21):7641–7646
Author information
Authors and Affiliations
Contributions
LYQ conceived idea, designed research and revised the manuscript; TKL and HM designed subsequent experiments, performed most of the in vitro and in vivo work, and wrote the manuscript; TF and XGG helped perform the in vivo experiments; YMF and ZYY assisted with interpretation of data and helped to perform the in vitro and in vivo work.
Corresponding author
Ethics declarations
Conflict of interest
All other authors have no conflicts of interest to disclose.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants
Written informed consent was obtained from each donor under a protocol approved by the Committee on Clinical investigation for the use of blood for cancer research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tao, K., He, M., Tao, F. et al. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol 82, 815–827 (2018). https://doi.org/10.1007/s00280-018-3670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3670-0