Abstract
Purpose
After approval of anti-programmed cell death (PD)-1 antibodies, treatment for non-small cell lung cancer (NSCLC) has drastically changed. However, even in patients with favorable effects, therapeutic efficacy does not last long. Recently, retreatment with anti-PD-1 antibody has received attention. The aim of this study was to evaluate the efficacy and safety of retreatment with pembrolizumab in NSCLC patients previously treated with nivolumab.
Patients and methods
We retrospectively reviewed NSCLC patients retreated with pembrolizumab who were previously treated with nivolumab. We collected the following data: patient characteristics, number of cycles of nivolumab and pembrolizumab, treatment interval between nivolumab and pembrolizumab, best response, and immune-related adverse events.
Results
Twelve patients were reviewed. The median number of cycles of nivolumab was 12.5 (range 2–32 cycles). Seven patients (58.3%) achieved a partial response (PR) and two patients (16.7%) achieved stable disease (SD). Eight patients (66.7%) received cytotoxic chemotherapy between nivolumab and pembrolizumab. The median number of cycles of chemotherapy treatment was 4 (range 1–9 cycles). The median number of cycles of pembrolizumab was 3.5 (range 1–17 cycles). One patient (8.3%) achieved PR and four patients (33.3%) achieved SD as their best response to pembrolizumab. All patients showing response to pembrolizumab had very high (≥ 80%) tumor PD-Ligand 1 expression.
Conclusions
This study suggested that retreatment with anti-PD-1 antibody is a reasonable option for selected NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, cancer immunotherapy has received remarkable attention, and the anti-programmed cell death (PD)-1 antibodies nivolumab and pembrolizumab have shown great success in multiple cancer types, including lung cancer. The CheckMate 017/057 and Keynote 010 studies showed a drastic advantage of nivolumab and pembrolizumab over docetaxel, a cytotoxic chemotherapy and the current standard of care for non-small cell lung cancer (NSCLC) [1,2,3]. The Keynote 024 study also showed superiority of pembrolizumab compared to platinum doublet as a first-line therapy [4]. On the basis of these studies, nivolumab and pembrolizumab have been approved and are available in the clinical setting for NSCLC patients in Japan. Immune checkpoint inhibitors such as nivolumab and pembrolizumab achieve drastic effect; however, not all patients see a response from immunotherapy, and those who gain no benefits instead receive conventional cytotoxic chemotherapy. Patients with disease progression after immune checkpoint inhibitors also generally receive conventional cytotoxic chemotherapy. The results of re-challenge of immune checkpoint inhibitors are largely unknown. Retreatment with anti-PD-1 antibodies in melanoma patients can re-establish disease control in selected patients [5, 6]. However, there are no reports of sequential use of immune checkpoint inhibitors in patients with NSCLC. The aim of this study was to evaluate the efficacy and safety of retreatment with pembrolizumab in patients with advanced NSCLC previously treated with nivolumab.
Patients and methods
This was a retrospective cohort study of NSCLC patients retreated with pembrolizumab after treatment with nivolumab at the National Hospital Organization Kyoto Medical Center (600-bed hospital) between December 2015 and March 2018. Patients were selected on the basis of the following two criteria: (1) pathologically confirmed NSCLC, and (2) retreatment with pembrolizumab after treatment with nivolumab. Patients received 2 mg/kg nivolumab every 2 weeks and 200 mg/body pembrolizumab every 3 weeks. We collected the following data: baseline characteristics, number of cycles of nivolumab and pembrolizumab, treatment regimens and cycles between nivolumab and pembrolizumab, best response to nivolumab and pembrolizumab therapy according to the Response Evaluation Criteria in Solid Tumors version 1.1 criteria, and immune-related adverse events (irAEs), which were assessed according to Common Terminology Criteria for Adverse Events version 4.0. We used the specimens obtained at initial diagnosis for evaluation of tumor PD-Ligand 1 expression by immunohistochemistry 22C3 pharmDx (Agilent, CA, USA). This study protocol was approved by the Ethical committee and the Institutional Review Board of National Hospital Organization Kyoto Medical Center.
Results
Patient characteristics
A total of 12 patients met the inclusion criteria. All patients had received nivolumab as the first anti-PD-1 antibody treatment, and pembrolizumab was administered to all patients as retreatment with anti-PD-1 antibody. Patient characteristics are shown in Table 1. The mean age at the induction of nivolumab was 70.8 ± 5.9 years. Of 12 patients, 7 had adenocarcinoma, and only 1 patient harbored an epidermal growth factor receptor mutation. All tumors were positive for PD-L1 expression. Of 12 patients, 5 (41.7%) had high [tumor proportion score (TPS) ≥ 50%] tumor PD-L1 expression and 7 patients (58.3%) had low (1% ≤ TPS < 50%) tumor PD-L1 expression. Outcomes between nivolumab and pembrolizumab treatment are shown in Table 2.
Outcomes after nivolumab treatment
Because nivolumab is approved for second- or later line therapy, all patients receiving nivolumab had received cytotoxic chemotherapy before nivolumab induction. The median number of regimens of cytotoxic chemotherapy before nivolumab was 2 (range 1–4), and all patients had a history of receiving platinum doublet chemotherapy.
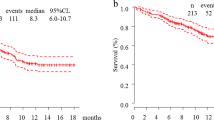
The median cycles of nivolumab were 12.5 (range 2–32 cycles), and the median progression-free survival (PFS) was 6.2 months (range 2.8–13.7 months). Of 12 patients, 7 (58.3%) achieved a partial response (PR) and 2 (16.7%) achieved stable disease (SD) as their best response. All patients discontinued nivolumab because of disease progression.
Treatment between nivolumab and pembrolizumab administration
Eight of 12 patients (66.7%) received cytotoxic chemotherapy between nivolumab and pembrolizumab treatment. Six of these patients (75.0%) received combination chemotherapy of docetaxel and ramucirumab. One patient received palliative radiotherapy with 45 Gy. The median number of cycles of treatment was 4 (range 1–9 cycles). Of 8 patients, 1 (12.5%) achieved PR and 4 (33.3%) achieved SD as their best response.
Outcomes after pembrolizumab treatment
The median number of cycles of pembrolizumab was 3.5 (range 1–17 cycles), and the median PFS was 3.1 months (range 1.2–12.6 months). Of 12 patients, 1 (8.3%) achieved PR and 4 (33.3%) achieved SD as their best response. Six (50.0%) patients showed no benefit from pembrolizumab. In 1 patient, best response and PFS were not evaluated because of early death after pembrolizumab. Of 6 patients with high PD-L1 expression (TPS ≥ 50%), 3 patients (50.0%) showed response (PR/SD). Of 5 patients with low PD-L1 expression (1% ≤ TPS < 50%), 2 patients (40.0%) showed response (SD). All patients showing response (PR and SD) during pembrolizumab had very high (TPS ≥ 80%) tumor PD-L1 expression.
Profiles of immune-related adverse events during first nivolumab and second pembrolizumab
IrAEs observed in patients receiving nivolumab and retreatment with pembrolizumab are shown in Table 3. Skin rash, infection, and interstitial pneumonia were most frequently observed. Two patients suffered grade 3 infection and grade 3 interstitial pneumonia during nivolumab treatment, but fully recovered after treatment for the irAEs. Two patients had herpes zoster viral infection and one patient had a methicillin-sensitive Staphylococcus aureus bloodstream infection.
Discussion
Recent advancements in cancer immunotherapy for lung cancer have provided valuable insight for the real world clinical setting. Unfortunately, previous clinical trials show that not every patient receives benefits from immunotherapy under the current circumstances. Clinicians can now use two anti-PD-1 antibodies, nivolumab and pembrolizumab, for the treatment of patients with NSCLC. However, whether anti-PD-1 antibodies have benefit as retreatment has not been examined.
In this study, 5 of 12 patients (41.7%) had disease stabilization during retreatment with pembrolizumab after treatment with nivolumab. This suggests that retreatment with anti-PD-1 antibodies could provide clinical benefit in selected patients, although it remains important to determine in which patients the retreatment might be effective.
In a previous report of melanoma patients, the response to the first anti-PD-1 antibody was suggested to be one of the parameters related to efficacy of retreatment [5]. In another report of patients with melanoma, PFS after the first anti-PD-1 treatment was a predictive factor of the efficacy of anti-PD-1 antibody retreatment [6]. The authors also mentioned the importance of the sequence of therapies [5, 6]. Immune checkpoint inhibitors tended to be more effective in melanoma patients with a history of receiving anti-cytotoxic T-lymphocyte antigen (CTLA)-4 antibody or radiation therapy. However, in our patients, the duration of PFS associated with nivolumab and treatment regimens between nivolumab and pembrolizumab administration showed no relation to the efficacy of pembrolizumab. Moreover, cytotoxic chemotherapy and radiation therapy between nivolumab and pembrolizumab administration did not affect the efficacy of pembrolizumab. Because previous studies examined melanoma patients, these results will not necessarily apply to lung cancer patients. Recently, it has been shown that radiation therapy plus immunotherapy has synergistic effects [7], known as “abscopal effects.” To evaluate the relationship between radiation therapy and immunotherapy, more large-scale studies should be conducted. Because other immune checkpoint inhibitors, such as anti-CTLA-4 antibody, are not used in lung cancer patients, it is unclear whether another immunotherapy affects the efficacy of retreatment with anti-PD-1 antibodies. This should also be evaluated in future studies.
PD-L1 expression is one of the biomarkers for predicting anti-PD-1 antibody efficacy. In previous large clinical trials, patients with high (TPS ≥ 50%) PD-L1 expression showed a favorable response [3, 4]. PD-L1 expression is now used as a companion diagnostic test in clinical settings as a predictive indicator of therapeutic efficacy to anti-PD-1 antibodies. In our study, all three patients with very high (TPS ≥ 80%) PD-L1 expression showed efficacy of retreatment with pembrolizumab. Patients with very high (TPS ≥ 80%) PD-L1 expression may receive benefit of anti-PD-1 antibody retreatment.
Our study raises the question of why the sequential use of anti-PD-1 antibodies shows benefit for selected patients. Answering this question remains difficult; however, some hypotheses have been suggested. Nivolumab and pembrolizumab are known to have different affinities for recombinant human PD-1 [8]. Pembrolizumab has higher affinity than does nivolumab, although it has recently shown that the two anti-PD-1 antibodies partially share a three-dimensional shape and mechanism, and are highly similar when interacting with PD-1 [9]. The two drugs have different dosages and administration intervals (nivolumab 2 mg/kg every 2 weeks versus pembrolizumab 200 mg/body fixed every 3 weeks) in the clinical setting. This difference may affect the therapeutic efficacy.
There are some limitations in this study. First, our study was retrospective and included a small sample size, which made statistical analysis difficult. Second, the timing and regimens prior to nivolumab treatment and between nivolumab and pembrolizumab treatment were chosen by the attending doctors, and therefore not standardized between patients. The selection of these chemotherapies might affect sequential anti-PD-1 antibody treatment. Third, we did not re-biopsy before pembrolizumab treatment, and tumor PD-L1 expression was only analyzed using the specimens taken at initial diagnosis. Therefore, the PD-L1 expression does not reflect the effect of the treatment with cytotoxic chemotherapy before nivolumab and between nivolumab and pembrolizumab.
In conclusion, we experienced 12 patients with NSCLC previously treated with nivolumab who were challenged to retreatment with pembrolizumab. Only selected patients received benefits of retreatment. Very high (TPS ≥ 80%) PD-L1 expression might be an indicator for anti-PD-1 antibody retreatment.
References
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR KEYNOTE-024 Investigators (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Blasig H, Bender C, Hassel JC, Eigentler TK, Sachse MM, Hiernickel J, Koop A, Satzger I, Gutzmer R (2017) Reinduction of PD1-inhibitor therapy: first experience in eight patients with metastatic melanoma. Melanoma Res 27:321–325
Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y, Matsumoto S, Muto M (2017) Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol 80:999–1004
Schoenhals JE, Seyedin SN, Tang C, Cortez MA, Niknam S, Tsouko E, Chang JY, Hahn SM, Welsh JW (2016) Preclinical rationale and clinical considerations for radiotherapy plus immunotherapy: going beyond local control. Cancer J 22:130–137
Fessas P, Lee H, Ikemizu S, Janowitz T (2017) A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol 44:136–140
Lee JY, Lee HT, Shin W, Chae J, Choi J, Kim SH, Lim H, Won Heo T, Park KY, Lee YJ, Ryu SE, Son JY, Lee JU, Heo YS (2016) Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat Commun 7:13354
Acknowledgements
The authors thank Miki Koda for her assistance of patient review and recording clinical data.
Funding
This study was partly supported by the National Hospital Organization fiduciary funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tadashi Mio has received honoraria from Bristol-Myers Squibb, Chugai Pharmaceutical, and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical approval
This study protocol was approved by the Ethical committee and the Institutional Review Board of National Hospital Organization Kyoto Medical Center.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fujita, K., Uchida, N., Kanai, O. et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol 81, 1105–1109 (2018). https://doi.org/10.1007/s00280-018-3585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3585-9