Abstract
Background
Melatonin has antitumor activity via several mechanisms including its antiproliferative and proapoptotic effects in addition to its potent antioxidant actions. Therefore, melatonin may be useful in the treatment of tumors in association with chemotherapy drugs.
Purpose and methods
This study was performed to study the role of melatonin receptors on the cytotoxicity and apoptosis induced by the chemotherapeutic agents cisplatin and 5-fluorouracil in two tumor cell lines, such as human colorectal cancer HT-29 cells and cervical cancer HeLa cells.
Results
We found that both melatonin and the two chemotherapeutic agents tested induced a decrease in HT-29 and HeLa cell viability. Furthermore, melatonin significantly increased the cytotoxic effect of chemotherapeutic agents, particularly, in 5-fluorouracil-challenged cells. Stimulation of cells with either of the two chemotherapeutic agents in the presence of melatonin further increased caspase-3 activation. Concomitant treatments with melatonin and chemotherapeutic agents augmented the population of apoptotic cells compared to the treatments with chemotherapeutics alone. Blockade of MT1 and/or MT2 receptors with luzindole or 4-P-PDOT was unable to reverse the enhancing effects of melatonin on both cytotoxicity, caspase-3 activation and the amount of apoptotic cells evoked by the chemotherapeutic agents, whereas when MT3 receptors were blocked with prazosin, the synergistic effect of melatonin with chemotherapy on cytotoxicity and apoptosis was reversed.
Conclusion
Our findings provided evidence that in vitro melatonin strongly enhances chemotherapeutic-induced cytotoxicity and apoptosis in two tumor cell lines, namely HT-29 and HeLa cells and, this potentiating effect of melatonin is mediated by MT3 receptor stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide, accounting for almost 10% of all cancers. According to the estimates, there were nearly 1.4 million new cases of CRC worldwide in 2012, and it is predicted that the number of cases diagnosed annually will rise to 2.4 million by 2035. Surgical excision is the main treatment and has proven to be particularly effective in CRC patients diagnosed at early stages (stage I and II). Nonetheless, recurrence after curative resection is a major issue, with recurrence rate being especially high in patients diagnosed at later stages of the disease (stage III and IV). Adjuvant chemotherapy, which is administered in first- and second-line treatment for advanced CRC, has demonstrated limited efficacy. Therefore, identification of new agents for their potential application in combined therapy for CRC treatment is warranted. On the other hand, cervical cancer is the second most common cause of female-specific cancer after breast cancer accounting for around 8% of both total cancer cases and total cancer deaths in women. Currently, surgery, radiotherapy, and cisplatin (CIS)-based chemotherapy are the primary methods for treating cervical cancer. Actually, neoadjuvant chemotherapy reduces the gross tumor volume, extends the 5 years survival rate, decreases the recurrence rate, and has thus attracted extensive attention for various studies [1].
Melatonin (N-acetyl-5-methoxytryptamine) is a low molecular weight indoleamine ubiquitously present in organisms from unicells to vertebrates. In the human, as in other vertebrates, melatonin is mainly synthesized in the pineal gland, although many other cells have now been shown to produce melatonin [2]. In the reproductive system, the ovary and the placenta produce melatonin using the same enzymatic machinery as that in the pineal gland [3].
Despite its simple chemical structure, melatonin is a pleiotropic molecule with remarkable biological functions from bacteria to mammals [4, 5]. In fact, this indoleamine governs biological rhythms [6], regulates reproduction [7], modulates the immune response [8], and controls the life/death balance of both tumor and normal cells [9,10,11,12], the latter mostly due to its ability to trigger survival- and apoptosis-promoting mechanisms, respectively. Moreover, not only melatonin but also its secondary, tertiary and quaternary metabolites have proven to be powerful antioxidants and free radical scavengers, a cascade reaction that is referred to as melatonin’s antioxidant cascade [13]. Nevertheless, melatonin may also exert its actions by binding specific receptors/interactors, such as the two well-characterized G-protein-coupled plasma membrane receptors MT1/MT2, or the quinone reductase II cytosolic enzyme, which has been previously defined as the MT3 receptor [14]. In addition, melatonin also possesses high affinity binding for nuclear receptors RORα/RZR, which act as transcriptional activators [15].
So far, various studies have reported that melatonin may be an excellent candidate as an anticancer agent or for combined therapy owing to its pro-oxidant, oncostatic, and pro-apoptotic activities in tumor cells [16]. Indeed, it has been previously described that treatment with the chemotherapeutic drug etoposide in combination with melatonin resulted in an increased elimination of leukemia cells derived from a patient with acute myeloid leukemia [15]. Similarly, in previous studies, we have verified the synergistic effect of melatonin on chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic carcinoma AR42J cells [17] and in human cervical cancer HeLa cells [18]. Preliminary clinical studies in patients with cancer also demonstrate the benefits of melatonin in association with cancer chemotherapeutic agents [19]. Moreover, a recent study has reported that melatonin acts as both a tumor metabolic inhibitor and circadian-regulated kinase inhibitor to promote the sensitivity of breast tumors to doxorubicin and drive tumor regression [20]. Therefore, melatonin may enhance the effectiveness of chemotherapy in terms of both tumor regression rate and survival time.
Few data are available on the effects of the therapeutic synergy of chemotherapeutic agents together with melatonin on human tumor cells. Hence, the present study investigated the in vitro effect of melatonin on chemotherapy-induced cytotoxicity and apoptosis in two tumor cell lines, such as human CRC HT-29 cells and cervical cancer HeLa cells, evaluating if the effects of melatonin are dependent on melatonin receptors. Particularly, we explored the anti-cancer effect of combined treatment with cisplatin (CIS) or 5-fluorouracil (5-FU) along with melatonin in HT-29 and HeLa cell lines. We found that the chemotherapy agents CIS and 5-FU succeeded in promoting cytotoxic and apoptosis-like actions towards HT-29 and HeLa cells. Interestingly, melatonin proved to be effective in enhancing the sensitivity of human tumor cells to chemotherapeutic agents, which is mediated by the signal transduction elicited by MT3 receptor stimulation.
Materials and methods
Reagents
HT-29 cell line (ECACC No. 91072201) derived from human colon adenocarcinoma and HeLa cell line (ECACC No. 93021013) derived from human epithelial cervix carcinoma were purchased from The European Collection of Cell Cultures (ECACC) (Dorset, UK). Fetal bovine serum (FBS) and penicillin/streptomycin were acquired from HyClone (Aalst, Belgium). L-Glutamine and Dulbecco’s Modified Eagle medium (DMEM) were obtained from Lonza (Basel, Switzerland). Cisdiammineplatinum (II) dichloride (cisplatin), 5-fluorouracil (5-FU), melatonin, HEPES, CHAPS, EDTA, dithiothreitol (DTT), N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (AC-DEVD-AMC), and 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were bought from Sigma-Aldrich (Madrid, Spain). 2-benzyl-N-acetyltryptamine (luzindole), 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine hydrochloride (prazosin), 4-cis-4-phenyl-2-propionamidotetralin (4-P-PDOT), N-(2-(6-chloro-5-methoxyindol-3-yl)ethyl)acetamide (6-chloromelatonin, CLM) and 8-methoxy-2-propionamidotetralin (8-M-PDOT) were purchased from Tocris Bioscience (Madrid, Spain). Annexin V-FITC apoptosis detection kit was acquired from eBioscience (Barcelona, Spain). All other reagents were of analytical grade.
Cell culture and treatment protocol
HT-29 and HeLa cells were grown in DMEM supplemented with 2 mM L-glutamine, 10% heat-inactivated FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin. Cells were cultured in a humidified atmosphere containing 95% air and 5% CO2 at 37 °C. Cells were routinely plated into 12-well plates at a density of 1 × 105 cells/mL, unless otherwise indicated, and the viability was > 95% in all experiments as assayed by the trypan-blue exclusion method. Alternatively, cells were visualized on a Nikon contrast phase microscope and photographed using a digital Nikon (DS-Qi1Mc) camera.
Cells were pre-treated for 30 min with 5 µM luzindole, 50 µM 4-P-PDOT or 10 nM prazosin, and then incubated with 20 μM CIS, 1 mM 5-FU, or the vehicle, in the absence or presence of 1 mM melatonin (or 100 nM of its agonists of melatonin MT1/MT2 receptors, namely 6-chloromelatonin (CLM) and 8-M-PDOT) for 48 h, unless otherwise specified. These particular concentrations of drugs were selected because they were previously reported to be effective in inducing cell death [9, 17, 18].
Cell viability assay
Cell viability was evaluated using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, which is based on the ability of viable cells to convert a water-soluble, yellow tetrazolium salt into a water-insoluble, purple formazan product. The enzymatic reduction of the tetrazolium salt happens only in living, metabolically active cells, but not in dead cells. Cells were seeded in 12-well plates at a density of 0.1 × 106 cells per well, and subsequently, exposed to the appropriate treatment at 37 °C. After the treatments, the medium was removed and MTT was added to each well, and then incubated for 60 min at 37 °C, as previously described [21]. The supernatant was discarded and DMSO was added to dissolve the formazan crystals. Treatments were carried out in triplicate. Optical density was measured in an automatic microplate reader (Infinite M200, Tecan Austria GmbH, Groedig, Austria) at a test wavelength of 490 nm and a reference wavelength of 650 nm to nullify the effect of cell debris. Data are presented as percentage above control (untreated samples).
Assay for caspase-3 activity
To determine caspase-3 activity, stimulated or resting cells (1.2 × 106 cells/mL) were sonicated and cell lysates were incubated with 2 mL of substrate solution (20 mM HEPES, pH 7.4, 2 mM EDTA, 0.1% CHAPS, 5 mM DTT and 8.25 µM caspase-3 substrate) for 1 h at 37 °C as previously described [22]. The activity of caspase-3 was calculated from the cleavage of the specific fluorogenic substrate (AC-DEVD-AMC). Substrate cleavage was measured with an automatic microplate reader (Infinite M200) with an excitation wavelength of 360 nm and emission at 460 nm. Preliminary experiments reported that caspase-3 substrate cleaving was not detected in the presence of the inhibitor of caspase-3, DEVD-CMK. Data were calculated as fluorescence units/mg protein and presented as fold increase over the pretreatment level (experimental/control).
Determination of apoptosis
Induction of apoptosis was determined using an Annexin V-FITC Apoptosis Detection Kit, according to manufacturer’s instructions. Briefly, stimulated or resting cells were harvested by trypsinization (1.2 × 106 cells/mL), washed twice with phosphate buffered saline (PBS), and centrifuged at 500 × g for 5 min; then the supernatant was discarded, and the pellet was resuspended in 200 µL binding buffer containing 5 µL of annexin V-FITC. Cells were incubated for 10 min at room temperature, washed twice, and finally resuspended in 200 µL binding buffer containing 10 µL of propidium iodide (PI). The cells were immediately analyzed after incubation with the probes by flow cytometry (Cytomics FC500; Beckman Coulter, Viena, Austria). Ten thousand events were analyzed using the FL-1 (green; annexin V-FITC) and FL-3 (red, PI) detectors. Each sample was tested from three to five times in independent experiments. Under all conditions tested, the percentages of annexin+/PI− (early apoptotic) and annexin+/PI+ (late apoptotic) cells were mainly compared [23].
Statistical analysis
Data are presented as mean ± standard error of mean (S.E.M) for each group. To compare the different treatments, statistical significance was calculated by one-way analysis of variance (ANOVA) followed by a post hoc Tukey test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Effect of melatonin receptor antagonists on cell viability
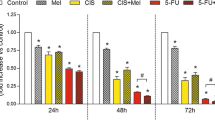
First, we analyze the effect of antagonists of melatonin receptors on cellular viability of tumor cells, i.e., HT-29 and HeLa cell lines. To do this, MTT assay was performed in the presence of different antagonists of melatonin receptors. Figure 1 shows the viability of human CRC HT-29 cells and cervical cancer HeLa cells challenged with melatonin in the absence and presence of antagonists of melatonin receptors. Stimulation of HT-29 (Fig. 1a, b) or HeLa (Fig. 1c, d) cells with 1 mM melatonin for 48 h resulted in a significant (P < 0.05) reduction of cell viability (75.8 ± 8.9%, Fig. 1a; and 73.8 ± 7.3%, Fig. 1c, respectively), as visualized with phase contrast microscopy, where it is observed that melatonin reduces both the number of HT-29 (Fig. 1b) and HeLa (Fig. 1d) cells in culture. To examine whether this effect of melatonin on cell viability was linked to melatonin receptors, we analyzed the effect of antagonists of melatonin receptors. For this purpose, cells were pre-treated with luzindole, which specifically antagonizes melatonin binding and activation of MT1/MT2 receptors; 4-P-PDOT, which selectively antagonizes MT2 receptors; or prazosin, a MT3 receptor antagonist. Pre-treatments of HT-29 (Fig. 1a, b) and HeLa (Fig. 1c, d) cells for 30 min with 5 µM luzindole or 50 µM 4-P-PDOT produced a negligible effect on cell viability induced by melatonin (78.2 ± 6.1 and 81.0 ± 6.8%, respectively, Fig. 1a; 77.0 ± 6.4 and 81.5 ± 5.7%, respectively, Fig. 1c), whereas pre-treatment of cells for 30 min with 10 nM prazosin significantly reversed the effect of melatonin on cellular viability (100.1 ± 5.8% in HT-29 cells, Fig. 1a; 100.7 ± 3.9% in HeLa cells, Fig. 1c, P < 0.05). These results were confirmed by morphological observations using phase contrast microscopy (Fig. 1b, d). Neither luzindole, nor 4-P-PDOT nor prazosin alone had any effect on the viability of HT-29 or HeLa cells (data not shown).
Effect of melatonin receptor antagonists on cell viability in HT-29 and HeLa cells. Cells were pre-treated for 30 min with 5 µM luzindole (LUZ), 50 µM 4-P-PDOT (4DOT) or 10 nM prazosin (PRAZ), or the vehicle, in the absence or presence of 1 mM melatonin (MEL) for 48 h. a, c Cell viability was evaluated by means of the MTT assay. Values are presented as means ± SEM of 4 independent experiments and expressed as percentage of control values (untreated cells). b, d Cells were visualized with phase contrast microscopy, and a representative field of each experimental group is shown. *P < 0.05 compared to control values. ● P < 0.05 compared to their corresponding value in the presence of MEL alone
Effect of melatonin on chemotherapy-induced cytotoxicity
We also explored the effects of co-treatment of HT-29 and HeLa cells with melatonin in the presence of two chemotherapeutic agents, such as CIS and 5-FU. Remarkably significant (P < 0.05) decreases in cell viability were observed upon stimulation of cells for 48 h with 20 μM CIS (Fig. 2a, b) or 1 mM 5-FU (Fig. 3a, c) (34.5 ± 9.1 and 30.7 ± 4.7%, respectively, in HT-29 cells; 37.9 ± 8.4 and 22.7 ± 3.9%, respectively, in HeLa cells). Similar results were observed in the photographs obtained with the phase contrast microscope, where it is observed that the treatment with CIS (Fig. 2b, d) or 5-FU (Fig. 3b, d) reduces the number of cells in culture, and therefore, the cell proliferation. In addition, parallel assays were carried out to examine the putative potentiating effect of melatonin on chemotherapy-induced cytotoxicity. Thus, when HT-29 and HeLa cells were incubated with the chemotherapy agents for 48 h in the presence of 1 mM melatonin, the indoleamine managed to further lower the cell viability of chemotherapy-challenged HT-29 cells, this effect being statistically significant (P < 0.05) for 5-FU-treated cells (11.1 ± 1.3% in HT-29 cells, Fig. 3a; 10.7 ± 1.9% in HeLa cells, Fig. 3c), however, no additional differences were noticed after 48 h of stimulation of cells with CIS (Fig. 2). We also evaluated the effect of antagonizing melatonin binding in the synergistic effect of melatonin on chemotherapy-induced cytotoxicity. As shown in Figs. 2 and 3, the blockade of MT1 and/or MT2 receptors with luzindole or 4-P-PDOT was unable to reverse the enhancing effects of melatonin on cytotoxicity evoked by CIS and 5-FU; whereas when MT3 receptors were blocked with prazosin, the synergistic effect of melatonin with chemotherapy was significantly (P < 0.05) reversed, particularly in cells treated with 5-FU (Fig. 3). Taken together, these results indicate that the potentiating effect of melatonin on the cytotoxic activity of the chemotherapeutic agents is mediated by the signal transduction elicited by MT3 receptor stimulation.
Effect of melatonin on cisplatin-induced cytotoxicity in HT-29 and HeLa cells. Cells were pre-treated for 30 min with 5 µM luzindole (LUZ), 50 µM 4-P-PDOT (4DOT) or 10 nM prazosin (PRAZ), or the vehicle, and then incubated with 20 μM cisplatin (CIS), or the vehicle (control), in the absence or presence of 1 mM melatonin (MEL) for 48 h. a, c Cell viability was evaluated by means of the MTT assay. Values are presented as means ± SEM of 5 independent experiments and expressed as percentage of control values (untreated cells). b, d Cells were visualized using phase contrast microscopy, and a representative field of each experimental group is shown. *P < 0.05 compared to control values
Effect of melatonin on 5-fluorouracil-induced cytotoxicity in HT-29 and HeLa cells. Cells were pre-treated for 30 min with 5 µM luzindole (LUZ), 50 µM 4-P-PDOT (4DOT) or 10 nM prazosin (PRAZ), or the vehicle, and then incubated with 1 mM 5-fluorouracil (5-FU), or the vehicle (control), in the absence or presence of 1 mM melatonin (MEL) for 48 h. a, c Cell viability was evaluated by means of the MTT assay. Values are presented as means ± SEM of 4–6 independent experiments and expressed as percentage of control values (untreated cells). b, d Cells were visualized with phase contrast microscopy, and a representative field of each experimental group is shown. * P < 0.05 compared to control values. ● P < 0.05 compared to their corresponding value in the presence of 5-FU alone. ■ P < 0.05 compared to their corresponding value in the presence of 5-FU + MEL alone
Effect of melatonin receptor agonists on chemotherapy-induced cytotoxicity
To verify that the synergistic effects of melatonin with the chemotherapeutic agents on cytotoxic activity are mediated by the activation only of the MT3 receptors, the cells were treated with 6-chloromelatonin (CLM) (a potent agonist with high affinity for melatonin MT1 and MT2 receptors) or 8-M-PDOT (an agonist selective for the melatonin MT2 receptor subtype). As shown in Fig. 4, treatment of HT-29 cells for 48 h with 100 nM CLM or 100 nM 8-M-PDOT in the presence of 20 µM CIS (Fig. 4a) or 1 mM 5-FU (Fig. 4b) were unable to modify the cytotoxic effect of both chemotherapeutic agents, indicating that the stimulation of the MT1 and MT2 receptors do not significantly participate in the potentiating effect of melatonin on the cytotoxic action of the two chemotherapeutic agents tested by us. Similar results were obtained in HeLa cells (Fig. 4c, d). Neither CLM nor 8-M-PDOT alone had any effect on the viability of HT-29 or HeLa cells (data not shown).
Effect of agonists of melatonin receptors on chemotherapy-mediated cytotoxicity in HT-29 and HeLa cells. Cells were treated with 20 μM cisplatin (CIS) (a, c) or 1 mM 5-fluorouracil (5-FU) (b, d), or the vehicle (control), in the absence or presence of 100 nM 6-chloromelatonin (CLM) or 8-M-PDOT (8DOT) for 48 h. Cell viability was evaluated by means of the MTT assay. Values are presented as means ± SEM of four independent experiments and expressed as percentage of control values (untreated cells). * P < 0.05 compared to control values
Effect of melatonin on chemotherapy-induced caspase-3 activation
To examine whether the potentiating effects of melatonin receptors and chemotherapeutic agents on cell viability were linked to apoptotic cell death, we analyzed the activation of caspase-3 (a key downstream effector of apoptosis) in both pre-treated with antagonists of melatonin receptors and resting (control) cells. Treatment of HT-29 cells with 1 mM melatonin for 48 h gave rise to a clear increase in caspase-3 activity (2.8 ± 0.2 fold-increase; P < 0.05; Fig. 5a, b). Additionally, treatments with 20 μM CIS or 1 mM 5-FU for 48 h also noticeably (P < 0.05) enhanced caspase-3 activity (2.3 ± 0.4 and 4.9 ± 0.5 fold-increase, respectively; Fig. 5a, b). Importantly, melatonin was able to enlarge chemotherapy-evoked caspase-3 activation. In fact, treatment of HT-29 cells for 48 h with 20 μM CIS (Fig. 5a) or 1 mM 5-FU (Fig. 5b) in the presence of 1 mM melatonin markedly (P < 0.05) triggered caspase-3 activation (5.4 ± 1.6 and 28.3 ± 4.8 fold-increase, respectively), with regards to the treatments with each chemotherapy agent alone. It is worth mentioning that 5-FU plus melatonin was the most effective chemotherapy agent in terms of caspase 3 activation, the stimulation being 23-fold higher than caspase basal activity (Fig. 5b). In HeLa cells, treatment for 48 h with both 1 mM melatonin and 20 μM CIS as well as 1 mM 5-FU were also able to increase significantly (P < 0.05) the enzymatic activity of caspase-3 (Fig. 5c, d), although HeLa cells showed greater sensitivity to CIS treatment (23.4 ± 4.7 fold-increase, Fig. 5c) than to 5-FU (5.8 ± 1.0 fold-increase, Fig. 5d). In a similar manner, treatment for 48 h of HeLa cells with 1 mM melatonin also potentiated significantly (P < 0.05) the enzymatic activity of caspase-3 caused by the doses of 20 μM CIS (Fig. 5c) and 1 mM 5-FU (Fig. 5d). Similarly, we also wanted to analyze the effect of antagonists of melatonin receptor on caspase-3 enzymatic activity in the presence of melatonin and the two chemotherapeutic agents. As shown in Fig. 5, pretreatments of HT-29 cells for 30 min with luzindole (a MT1/MT2 receptor antagonist) or 4-P-PDOT (which selectively antagonizes MT2 receptor) were unable to reverse the enhancing effects of melatonin on caspase-3 activity evoked by CIS and 5-FU; whereas when MT3 receptors were blocked with prazosin, the synergistic effect of melatonin with chemotherapy was significantly (P < 0.05) reversed, both in HT-29 (Fig. 5a, b) and HeLa (Fig. 5c, d) cells.
Effect of melatonin on chemotherapy-mediated caspase-3 activation in HT-29 and HeLa cells. Cells were pre-treated for 30 min with 5 µM luzindole (LUZ), 50 µM 4-P-PDOT (4DOT) or 10 nM prazosin (PRAZ), or the vehicle, and then incubated with 20 μM cisplatin (CIS) or 1 mM 5-fluorouracil (5-FU), or the vehicle (control), in the absence or presence of 1 mM melatonin (MEL) for 48 h. Caspase-3 enzymatic activity was estimated as described under "Materials and methods". Values are presented as means ± SEM of 6–7 independent experiments and expressed as fold increase over the pre-treatment level (experimental/control). * P < 0.05 compared to control values. ● P < 0.05 compared to their corresponding value in the presence of CIS or 5-FU alone. ■ P < 0.05 compared to their corresponding value in the presence of CIS + or 5-FU + MEL alone
Effect of melatonin on chemotherapy-mediated apoptotic cell death
To evaluate cells’ apoptotic stage following stimulation with CIS or 5-FU in the presence of melatonin, we analyzed the redistribution of phosphatidylserine (PS) in the presence of PI. Figures 6 and 7 portray representative cytograms for each treatment assayed. Stimulation of HT-29 (Fig. 6) and HeLa (Fig. 7) cells with 1 mM melatonin alone for 48 h produced a significant (P < 0.05) diminution in the percentage of alive cells (annexin−/PI−; 64.5 ± 2.6%, Fig. 6b; and 63.6 ± 3.6%, Fig. 7b) and increased slightly both the proportion of early apoptotic cells (annexin+/PI−; 17.3 ± 2.5%, Fig. 6b; and 10.9 ± 2.7%, Fig. 7b), and the amount of late apoptotic cells (annexin+/PI+; 11.1 ± 2.1%, Fig. 6b; and 11.2 ± 1.5%, Fig. 7b). Treatments of HT-29 cells (Fig. 6b) with 20 μM CIS for 48 h caused a slight increase in the percentage of early (19.5 ± 3.8%) and late (10.1 ± 2.9%) apoptotic cells at the expense of the amount of alive cells, which was significantly (P < 0.05) decreased (63.7 ± 3.7%). Similar results were obtained when HeLa cells were incubated in the presence of CIS, although a greater increase was observed in the percentage of late apoptotic cells (25.7 ± 2.0%; Fig. 7b). Of note, simultaneous administration of 20 μM CIS and 1 mM melatonin for 48 h in both HT-29 (Fig. 6) and HeLa (Fig. 7) cells brought about an augmentation (P < 0.05) in the number of early (28.7 ± 2.8%, Fig. 6b; and 22.5 ± 1.5%, Fig. 7b) and late (16.5 ± 3.1%, Fig. 6b; and 27.5 ± 1.9%, Fig. 7b) apoptotic cells. As far as 5-FU is concerned, stimulation of HT-29 (Fig. 6) and HeLa (Fig. 7) cells with 1 mM 5-FU for 48 h significantly (P < 0.05) increased the percentage of both early (22.6 ± 2.2%, Fig. 6b; and 13.3 ± 3.9%, Fig. 7b) and late (23.4 ± 2.1%, Fig. 6b; and 34.2 ± 3.2%, Fig. 7b) apoptotic cells, as well as a concomitant decrement in the proportion of living cells (52.6 ± 4.4%, Fig. 6b; and 44.5 ± 4.0%, Fig. 7b). Moreover, concomitant stimulation of HT29 (Fig. 6) and HeLa (Fig. 7) cells with 1 mM 5-FU and 1 mM melatonin for 48 h significantly (P < 0.05) potentiated the killing ability of 5-FU, especially in the amount of early apoptotic both HT-29 (33.1 ± 2.9%, Fig. 6b) and HeLa cells (30.3 ± 3.9%, Fig. 7b) and late apoptotic in HT-29 cells (38.2 ± 4.0%, Fig. 6b). These results are in agreement with our previous findings on caspase-3 activation, i.e., melatonin somehow accelerated chemotherapy-induced apoptosis.
Effect of melatonin on chemotherapy-mediated apoptotic cell death in HT-29 cells. Cells were pre-treated for 30 min with 10 nM prazosin (PRAZ), or the vehicle, and then incubated with 20 μM cisplatin (CIS) or 1 mM 5-fluorouracil (5-FU), or the vehicle (control), in the absence or presence of 1 mM melatonin (MEL) for 48 h. Apoptotic populations were detected by flow cytometry, as described under "Materials and methods". a Representative plots depicting the redistribution of phosphatidylserine (annexin V staining) in the presence of propidium iodide (PI) after 48 h of treatment with the indicated combination of drugs. b Histograms showing percentages of each cell population. Values are presented as means ± SEM of 3–5 independent experiments. *P < 0.05 compared to respective control values. ● P < 0.05 compared to their corresponding value in the presence of CIS or 5-FU alone. ■ P < 0.05 compared to their corresponding value in the presence of CIS + MEL or 5-FU + MEL alone
Effect of melatonin on chemotherapy-mediated apoptotic cell death in HeLa cells. Cells were pre-treated for 30 min with 10 nM prazosin (PRAZ), or the vehicle, and then incubated with 20 μM cisplatin (CIS) or 1 mM 5-fluorouracil (5-FU), or the vehicle (control), in the absence or presence of 1 mM melatonin (MEL) for 48 h. Apoptotic populations were detected by flow cytometry, as described under "Materials and methods". a Representative plots depicting the redistribution of phosphatidylserine (annexin V staining) in the presence of propidium iodide (PI) after 48 h of treatment with the indicated combination of drugs. b Histograms showing percentages of each cell population. Values are presented as means ± SEM of 3–5 independent experiments. *P < 0.05 compared to respective control values. ● P < 0.05 compared to their corresponding value in the presence of CIS or 5-FU alone. ■ P < 0.05 compared to their corresponding value in the presence of CIS + MEL or 5-FU + MEL alone
Finally, to further confirm the participation of melatonin MT3 receptors on chemotherapeutics-mediated apoptosis, cells were pre-treated again with the MT3 receptor antagonist prazosin. As shown in Figs. 6 and 7, prazosin reversed the synergistic actions of melatonin with chemotherapeutic agents on apoptosis. In fact, pretreatments of HT-29 (Fig. 6) and HeLa (Fig. 7) cells for 30 min with 10 nM prazosin increased the proportion of living cells, while the amount of early and late apoptotic cells was significantly (P < 0.05) decreased, when compared with the results obtained in the presence of melatonin plus CIS or 5-FU.
Discussion
Apoptosis or programmed cell death is a fundamental physiological process that plays a major role in tissue homeostasis, organ development and elimination of defective or potentially dangerous cells [24]. However, any defect in the control of apoptosis may lead to pathological conditions such as cancer when proliferation is increased, i.e., when the rate of apoptosis is downregulated, while it may cause neurodegenerative and autoimmune diseases when the rate of cell death is upregulated. As for melatonin, it is a multitasking molecule that employs a variety of mechanisms to modulate the physiology and molecular biology of cells [25]. Nonetheless, in the last few years, melatonin has attracted much attention due to its influence on the apoptotic process. The precise mechanism whereby melatonin regulates apoptosis still remains unknown, although it has been described that the indoleamine possesses both pro- and anti-apoptotic actions depending on cell type [26]. In the last decade a novel effect of melatonin on apoptosis was reported, where melatonin has been shown to protect normal cells from apoptosis [22]; conversely, in various tumor cells it was found to induce apoptosis [for review see 26]. For example, melatonin reportedly promotes apoptotic cell death in several tumor cells including human myeloid HL-60 cells [9], B-lymphoma cells [27], HT-29 human CRC cells [28], and rat pituitary prolactin-secreting tumor cells [29].
Herein, we investigated the putative potentiating effect of melatonin on chemotherapy-induced cytotoxicity and apoptosis in two tumor cell lines, such as human colorectal adenocarcinoma HT-29 cells and cervical cancer HeLa cells. We reported that melatonin per se is able to display cytotoxic and pro-apoptotic actions towards HT-29 and HeLa cells. Similar results have been previously shown by our group [9, 21] and others [30,31,32]. Most importantly, the indoleamine proved to be effective in improving the tumor killing abilities of two chemotherapy agents, namely cisplatin (CIS) and 5-fluorouracil (5-FU). For instance, we observed that the indoleamine managed to further enhance 5-FU-induced caspase-3 activation, the stimulation being 28-fold higher than caspase basal activity (23-fold higher than 5-FU-triggered caspase-3 activity). Apart from this, melatonin only displayed mild or moderate chemosensitizing effects on cytotoxicity activity in CIS-challenged HT-29 and HeLa cells. This may be due to a stimulation of caspases without the need to lead to cell death by apoptosis. This apoptosis independent of caspase stimulation has been previously described and has been shown to be required for certain cellular functions, including store-operated calcium entry, platelet aggregation or pancreatic secretion [33]. In this sense, previous studies have proved that melatonin strengthens the effect of other chemotherapeutic agents, such as doxorubicin or puromycin, in various tumor cell lines, including human Ewing sarcoma cancer cells [34], human hepatoma cell lines [35], human leukemia cell line HL-60 [36], as well as human lung cancer cell line A-549 [37]. More recently, it has been shown that the enhanced anticancer effect of melatonin and 5-FU in CRC cells is mediated through modulation of caspase-dependent apoptosis [38].
The potentiating actions of melatonin on chemotherapy-stimulated apoptosis in HT-29 and HeLa cells were further confirmed after analyzing the populations of apoptotic cells via annexin V/PI. In this way, when compared to CIS or 5-FU alone, simultaneous administration of these chemotherapeutic agents together with melatonin brought about a substantial improvement in the number of early (annexin+/PI−) and late (annexin+/PI+) apoptotic cells as well as a concomitant decrement in the proportion of living cells (annexin−/PI−), particularly in 5-FU-treated cells. In this regard, previous studies have verified the synergistic effect of melatonin on chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic carcinoma AR42J cells [17]. Similarly, other authors have recently highlighted the synergistic effect of melatonin on doxorubicin-evoked apoptosis in the human hepatoma cell line HepG2 [26].
To sum up, our findings provided evidence that in vitro melatonin strongly enhanced the sensitivity of human colon adenocarcinoma and cervical carcinoma cells to CIS and 5-FU. So far, synergistic antitumor actions of melatonin are still controversial and seem to be dependent on the chemotherapy agent used and the tissue where cancer cells were derived from. In fact, it has been described that melatonin, in opposition to what is expected, lessens idarubicin-elicited nuclear fragmentation in both healthy lymphocytes and leukemic K562 cells [39]. Likewise, melatonin has been proven to attenuate anti-tumor actions of CIS in human liver carcinoma HepG2 cells via the modulation of the balance of apoptotic proteins [40]. Other studies have also indicated that melatonin does not interfere with the cytotoxic effect of chemotherapy agents such as cytarabine, daunorubicin and etoposide in different leukemia cell lines, including Jurkat, MOLT-4, Daudi, HL-60, CMK, and K562 [16]. Despite this, it has been recently demonstrated that melatonin enhances CIS-induced cytotoxicity in different human ovarian cancer cells, namely, SK-OV-3 [41], HTOA and OVCAR-3 [42], which consistently agrees with our own results in human CRC HT29 cells. Therefore, findings on the in vitro chemosensitizing effect of melatonin in female genital tract malignancies seem to be pretty consistent, thereby suggesting that the indoleamine could be potentially applied as a coadjuvant agent to improve the curative effect of chemotherapy, particularly of platinum-based therapy, on tumors affecting the female reproductive system.
Finally, melatonin, as a powerful antioxidant [11, 13], displays protective actions against chemotherapy which may be due, at least in part, to a reduction in the overproduction of ROS in healthy cells [13]. Nevertheless, stunning findings have recently indicated that melatonin may behave as a pro-oxidant molecule in tumor cells [13, 19]. Many of the effects of melatonin in mammals are mediated through interaction with the G-protein coupled membrane bound melatonin receptors type 1 and type 2 (MT1 and MT2, respectively) or, indirectly with nuclear orphan receptors from the RORα/RZR family. Melatonin also binds to the quinone reductase II cytosolic enzyme, previously defined as the MT3 receptor [14, 15]. In fact, solid data by Tan et al. [43] support the idea that the MT3 melatonin binding site is the enzyme quinone reductase II, rather than a membrane melatonin receptor. Our data provide for the first time evidence for the involvement of the MT3 melatonin receptor in the augmentation of cytotoxic and pro-apoptotic actions induced by the chemotherapy agents CIS and 5-FU. When the MT1 and/or MT2 receptors are blocked by the administration of luzindol and 4-P-PDOT, no modifications were observed in the enhancing effects of melatonin and the two chemotherapeutic agents either on cytotoxic activity, the activity of caspase-3 or amount of apoptotic cells; whereas only in the presence of prazosin (a melatonin MT3 receptor antagonist) the synergistic actions of melatonin with the chemotherapeutic agents were clearly reversed. These results were confirmed since in the presence of melatonin MT1 and MT2 receptor agonists, where the effects of the chemotherapeutic agents were not modified. Additionally, we cannot discard the possibility that the antioxidant activity of melatonin may, in fact, relate at least in part, to the interaction of melatonin with quinone reductase 2 (the putative MT3 melatonin receptor), as recently described [44]. Taken together, these results clearly indicate that the enhancing effects that melatonin exerts on the cytotoxic and pro-apoptotic activity of CIS and 5-FU are mediated by the activation of melatonin MT3 receptors, at least in HT-29 and HeLa cells.
In conclusion, we provide evidence that in vitro melatonin strengthens chemotherapeutic-stimulated cytotoxicity and apoptosis in HT-29 and HeLa cells through the stimulation of MT3 melatonin receptors, therefore, the indoleamine could be potentially applied to cancer treatment as an adjuvant agent. Given that melatonin has been reported to inhibit tumor growth and progression in animal models of cancer [45], it would be thus interesting to perform further in vivo studies to corroborate the results obtained in the present research.
References
Okazawa M, Mabuchi S, Isohashi F et al (2013) Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage IB1-IIB cervical cancer patients with intermediate-risk or high-risk factors: a 13-year experience. Int J Gynecol Cancer 23:567–575
Reiter RJ, Tan DX, Rosales-Corral SA, Manchester LC (2013) The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 13:373–384
Reiter RJ, Tan DX, Tamura H, Cruz MH, Fuentes-Broto L (2014) Clinical relevance of melatonin in ovarian and placental physiology. Gynecol Endocrinol 30:83–89
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93:350–384
Meng X, Li Y, Li S et al (2017) Dietary sources and bioactivities of melatonin. Nutrients 9:367. doi:10.3390/nu9040367
Arendt J, Skene DJ (2005) Melatonin as a chronobiotic. Sleep Med Rev 9:25–39
Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol Reprod 81:445–456
Calvo JR, Gonzalez-Yanes C, Maldanado MD (2013) The role of melatonin in the cells of the innate immunity: a review. J Pineal Res 55:103–120
Bejarano I, Redondo PC, Espino J et al (2009) Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL 60 cells. J Pineal Res 46:392–400
Espino J, Bejarano I, Redondo PC et al (2010) Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J Membr Biol 233:105–118
Espino J, Ortiz A, Bejarano I et al (2011) Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil Steril 95:2290–2296
Zhang JJ, Meng X, Li Y et al (2017) Effects of melatonin on liver injures and diseases. Int J Mol Sci 18:673. doi:10.3390/ijms18040673
Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42:28–42
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–166
Wiesenberg L, Missbach M, Carlberg C (1998) The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci 12:143–150
Reiter RJ, Tan DX, Sainz R, Mayo JC, Lopez-Burillo S (2002) Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol 54:1299–1321
Uguz AC, Cig B, Espino J et al (2012) Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res 53:91–98
Pariente R, Pariente JA, Rodríguez AB, Espino J (2016) Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res 60:55–64
Lissoni P (2007) Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol 55:201–204
Xiang S, Dauchy RT, Hauch A et al (2015) Doxorubicin resistance in breast cancer is driven by light at night induced disruption of the circadian melatonin signal. J Pineal Res 59:60–69
Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2011) Pro-oxidant effect of melatonin in human leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol 108:14–20
Espino J, Bejarano I, Paredes SD, Barriga C, Rodríguez AB, Pariente JA (2011) Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J Pineal Res 51:195–206
Espino J, Rodríguez AB, Pariente JA (2013) The inhibition of TNF-α-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J Pineal Res 54:442–452
Strasser L, O´Connor VM, Dixit (2000) Apoptosis signaling. Ann Rev Biochem 69:217–245
Reiter RJ, Tan DX, Fuentes-Broto L (2010) Melatonin: a multitasking molecule. Prog Brain Res 181:127–151
Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ (2003) Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci 60:1407–1426
Trubiani O, Recchuoni R, Moroni F et al (2005) Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathways activation. J Pineal Res 39:425–431
García-Navarro A, González-Puga C, Escames G et al (2007) Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J Pineal Res 43:195–205
Yang QH, Xu JN, Xu RK, Pang SF (2007) Antiproliferative effects of melatonin on the growth of rat pituitary prolactin-secreting tumor cells in vitro. J Pineal Res 42:131–138
Rubio S, Estevez F, Cabrera J, Reiter RJ, Loro J, Quintana J (2007) Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J Pineal Res 42:131–138
Cos S, García-Bolado A, Sánchez-Barceló EJ (2001) Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16B16 and PG19). Melanoma Res 11:197–201
Li Y, Li S, Zhou Y et al (2017) Melatonin for prevention and treatment of cancer. Oncotarget 8:39896–39921
Rosado JA, Lopez JJ, Gomez-Arteta E, Redondo PC, Salido GM, Pariente JA (2006) Early caspase-3 activation independent of apoptosis is required for cellular function. J Cell Physiol 209:142–152
Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G (2010) Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res 48:72–80
Fan LL, Sun GP, Wang ZG (2010) Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol 16:1473–1481
Koh W, Jeond SJ, Lee HJ (2011) Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5′-adenosine monophosphate-activated kinase-alpha in human leukaemia HL-60 cells. J Pineal Res 50:367–373
Fic M, Podhorska-Okolow M, Dziegiel P et al (2007) Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A-549, laryngeal cancer cell line Hep-2). In Vivo 21:513–518
Gao Y, Xiao X, Zhang C et al (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal Res. doi:10.1111/jpi.12380
Majesterek I, Glock E, Blasiak J et al (2005) A comparison of the action of amifostine and melatonin on DNA-damaging effects and apoptosis induced by idarubicin in normal and cancer cells. J Pineal Res 38:254–263
Bennukul K, Numkliang S, Leardkamolkarn V (2014) Melatonin attenuates cisplatin-induced HepG2 cell death via the regulation of mTOR and ERCC1 expressions. World J Hepatol 6:230–242
Kim JH, Jeong SJ, Kim B et al (2012) Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res 52:244–252
Futagami M, Sato S, Sakamoto T et al (2001) Effects of melatonin on the proliferation and cis-diamminedichloroplatinum (CDDP) sensitivity of cultured human ovarian cancer cells. Gynecol Oncol 82:544–549
Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ (2007) Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J Pineal Res 43:317–320
Boutin JA, Bonnaud A, Brasseur C et al (2017) New MT2 melatonin receptor-selective ligands: agonists and partial agonists. Int J Mol Sci 18:1347. doi:10.3390/ijms18071347
Paroni R, Terraneo L, Bonomini F et al (2014) Antitumour activity of melatonin in a mouse model of human prostate cancer: relationship with hypoxia signaling. J Pineal Res 57:43–52
Acknowledgements
The authors appreciate the technical and human support provided by the facility of Bioscience Applied Techniques of SAIUEx (financed by UEx, Junta de Extremadura, MICINN, FEDER, and FSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Pariente declares that he has no conflict of interest. I. Bejarano declares that he has no conflict of interest. J. Espino declares that he has no conflict of interest. A.B. Rodriguez declares that she has no conflict of interest. J.A. Pariente declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This work was supported by Gobierno de Extremadura grants (GR15051). J. Espino holds a research post-doctoral fellowship from Gobierno de Extremadura (jointly financed by the European Regional Development Fund (ERDF); ref. PO14011).
Rights and permissions
About this article
Cite this article
Pariente, R., Bejarano, I., Espino, J. et al. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother Pharmacol 80, 985–998 (2017). https://doi.org/10.1007/s00280-017-3441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3441-3