Abstract
Purpose
To evaluate the efficacy and safety of gemcitabine plus cisplatin in Japanese patients with unresectable gallbladder cancer (GBC).
Methods
Chemo-naïve patients with histologically proven unresectable GBC were enrolled in this study. The patients received gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) on days 1 and 8, every 21 days. A response assessment was done by CT scan every 4 weeks. The primary end points were to determine the response rates [RR; complete response (CR) + partial response (PR)] and the disease control rate [DCR; CR + PR + stable disease (SD)]. The secondary end points were to evaluate toxicity, progression-free survival (PFS), and overall survival (OS).
Results
From March 2012 to February 2015, 14 patients from seven different institutions were enrolled in the study, and 13 cases were evaluable for assessment. Eleven cases (84.6%) had distant metastases, and 8 cases (61.5%) had obstructive jaundice. There was no CR, 1 PR (7.7%), 11 SD (84.6%), and 1 progressive disease (PD) (7.7%). The RR was 7.7%, whereas the DCR was 92.3%. The median PFS was 3.1 months, the median OS was 6.2 months, and the one-year survival rate was 0%. Grade 3 hematologic toxicity was observed in three cases (23%), but all of them recovered upon drug withdrawal, and there was no treatment-related death.
Conclusion
Although gemcitabine plus cisplatin has a high DCR (92.3%) and relatively low toxicity, the RR is less than 10%, and development of new therapies is desired for the treatment of unresectable GBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite that it is relatively rare, the incidence of biliary tract cancer (BTC) varies widely in different geographic regions, with the lowest incidence rates in Western countries and the highest rates in Latin America and Asia including Japan [1]. The number of deaths due to BTC was 18,152 in 2015 (Demographic Statistics in Japan), making this cancer the sixth leading cause of cancer death in Japan.
Gallbladder cancer (GBC) is the most common malignancy in BTC. Surgery offers the only chance of a cure, but most of the patients present with advanced disease and are only candidates for palliative treatment. The prognosis for patients with advanced GBC is worse than for those with BTC originating at other sites, with a median survival of 2.8 months if untreated, compared with 5.5–10.1 months for untreated cholangiocarcinoma [2].
Gemcitabine has been widely evaluated for patients with unresectable BTC. Recently, combination therapies based on gemcitabine have been considered to enhance the effectiveness of gemcitabine. Currently, a regimen of gemcitabine combined with platinum is recommended as a standard chemotherapy for patients with unresectable BTC, given that the phase III trial from the UK published in the New England Journal of Medicine compared gemcitabine plus cisplatin (GC) to gemcitabine alone in 410 patients with locally advanced or metastatic BTC [3]. However, carcinomas of the biliary tract and gallbladder may behave differently with respect to the biological behavior and the response to treatment [4], and there have been very few studies on the role of palliative chemotherapy exclusively enrolling GBC patients. We conducted a prospective multicenter phase II study to investigate the efficacy and safety of GC in chemo-naïve, unresectable GBC patients without previous surgery in Japan.
Materials and methods
Study design
This was a prospective multicenter study. All patients provided written informed consent. A total of seven centers participated in this study (Nagoya University Hospital, Daido Hospital, Ogaki Municipal Hospital, Komaki City Hospital, Okazaki City Hospital, Toki General Hospital, and Tokai Central Hospital). This study was approved by the institutional review board at each center and was conducted in accordance with the Declaration of Helsinki. This study was also registered as UMIN000001267 (UMIN Clinical Trials Registry (UMIN-CTR)).
Inclusion and exclusion criteria
The following inclusion criteria were used for the selection of the patients: histologically or cytologically proven gallbladder adenocarcinoma; unresectable locally advanced or metastatic disease; at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria; no previous chemotherapy or radiotherapy; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1; an estimated life expectancy of more than 3 months; older than 20 years of age; adequate bone marrow function (white blood cell count ≧3000/mm3, neutrophil count ≧1500/mm3, platelet count ≧100,000/mm3, hemoglobin level ≧10.0 g/dl); adequate renal function [creatinine clearance (Cockroft–Gault Equation) ≧60 ml/min]; and adequate liver function (serum total bilirubin ≦2 times the upper limits of normal (ULN), transaminases ≦3 times ULN).
The exclusion criteria were as follows: interstitial pneumonia or pulmonary fibrosis; uncontrollable diabetes mellitus; liver function disorder; cardiac angina or cardiac infarction within 3 months; active infection; symptomatic brain metastases; other active malignancies; ascites or edema, which requires treatment; pregnant or lactating women; severe drug-induced allergy; and other severe complications.
Treatment
Each cycle comprised cisplatin (25 mg per square meter of body surface area) followed by gemcitabine (1000 mg per square meter), and each was administered on days 1 and 8 every 3 weeks. Gemcitabine plus cisplatin was administered as a 2-h infusion (1 L of 0.9% saline including cisplatin, 20 mmol potassium chloride, and 8 mmol magnesium sulfate over 1 h followed by 500 ml of 0.9% saline over 30 min before the administration of gemcitabine). The treatment was given for a minimum of one cycle and continued for a maximum of 16 cycles, unless disease progression was evident, an intolerable adverse event occurred, or the patient was required to withdraw from the study.
Subsequent treatment cycles were started only under the following conditions: neutrophil count ≧1500/mm3; platelet count ≧100,000/mm3; creatinine clearance (Cockroft–Gault Equation) ≧60 ml/min; serum total bilirubin ≦3 times ULN; and transaminases ≦5 times ULN. The protocol was temporarily discontinued when any of the following conditions were encountered until the resolution of toxicity or return of toxicity to the conditions upon commencement of the treatment: neutrophils <1000/mm3; platelets <7000/mm3; creatinine clearance (Cockroft–Gault Equation) <60 ml/min; serum total bilirubin >3 times ULN; transaminases >5 times ULN; grade 3 or 4 non-hematological toxicity other than anorexia; fatigue; or alopecia. The gemcitabine dose of the subsequent course was reduced to 800 mg/m2 for cases of grade 4 neutropenia (<500/mm3), thrombocytopenia (<20,000 mm3), or transaminases >10 times ULN.
Palliation of obstructive jaundice and gastric outlet obstruction were done by an interventional approach wherever feasible.
Efficacy assessment
All patients who received at least one dose of the study drug were included in the efficacy and safety assessment. The response assessment [complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)] was done by a computed tomography (CT) scan, which was performed at least once every 4 weeks according to the RECIST criteria. A CR was defined as the disappearance of all known disease determined by two observations not less than 3 weeks apart. A PR was defined as at least a 30% decrease in measurable disease by two observations not less than 3 weeks apart and no evidence of any new lesions or progression of any existing lesions. An inability to demonstrate a 30% decrease in tumor size or a 20% increase in the size of one or more lesions, as well as no new lesions for more than 6 weeks, was defined as SD. A 20% increase in the size of one or more measurable lesions or the appearance of any new lesions was defined as PD. Patients who developed progressively increasing jaundice during the follow-up were also considered to have PD.
Statistics
The primary end point was to measure the response rate (RR), defined as CR + PR. In this study, the disease control rate (DCR), including SD in the RR, was also investigated. The secondary end points were the safety (frequency and degree of toxicity) of this protocol, progression-free survival (PFS), and overall survival (OS). The adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0). The PFS was measured from the date of enrollment until the date of PD. The OS was calculated from the date of enrollment until the date of death. The Kaplan–Meier survival analysis method was used to estimate the median OS, as well as the PFS, and the 95% confidence intervals (CIs) were calculated. A log rank test was used to compare the differences among the patients grouped based upon the individual covariates. A Cox proportional hazard model was used for a multivariate analysis of overall survival. The statistics are presented using the median (with IQR) for the quantitative variables, and the categorical variables are presented in frequencies along with the respective percentages. A P value of 0.05 or lower was considered to be statistically significant. All statistical analyses were performed by using SPSS software (Version 24, SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
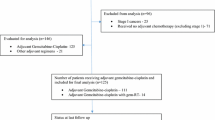
From March 2012 to February 2015, 14 patients from seven different institutions were enrolled in the study, and 1 patient was excluded because the transaminases were >5 times ULN prior to treatment. The remaining 13 patients were eligible for efficacy and safety assessment. The ratio of male to female patients was 6:7. The patient characteristics are shown in Table 1. In total, 10 patients (76.9%) had an ECOG performance status of 0, 11 patients (84.6%) had metastatic disease, and 8 (61.5%) patients initially presented with obstructive jaundice.
Efficacy assessment
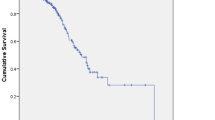
No patient achieved a CR, 1 patient (7.7%) had a PR, 11 patients (84.6%) had SD, and 1 patient (7.7%) showed PD. The RR (CR + PR) to chemotherapy was 7.7%, and the DCR (CR + PR + SD) was 92.3% (Table 2). The only patient who showed a PR was a locally advanced GBC with no metastasis or jaundice at the initial diagnosis. He had a total of seven cycles of GC, and the duration of the PR was 9 weeks. The median cycles of GC given was four (IQR 1.5). Temporary discontinuation of the protocol occurred in six cases (46%), but no case had a dose reduction. The median PFS was 3.1 months (95% CI 2.8–3.5) (Fig. 1), the median OS was 6.2 months (95% CI 5.1–7.2), and 1-year overall survival was 0% (Fig. 2). The number of chemotherapy cycles given was a significant individual prognostic factor affecting the OS (Table 3). The Cox proportional hazards regression analysis indicated a significant effect of not less than four cycles of GC (P = 0.017) given by the OS of these patients (Table 4; Fig. 3).
Safety assessment
The toxicities observed during the treatment are listed in Table 5. Grade 3 toxicities were seen in three patients (23%), neutropenia in two patients, and a hemoglobin decrease and thrombocytopenia in one patient. Grade 4 toxicities were not observed during this study, and there were no treatment-related deaths. All of the patients recovered from the above adverse events by discontinuing the treatment.
Eleven patients (84.6%) continued GC until PD, and the remaining two patients discontinued treatment due to uncontrollable cholangitis and renal impairment. Seven patients (53.8%) received second-line chemotherapy after failure of GC with S-1 (tegafur/gimeracil/oteracil potassium) (five patients) and gemcitabine alone (two patients).
Discussion
In this prospective multicenter study investigating the efficacy and safety of GC in patients with unresectable GBC in Japan, although we achieved a DCR of 92.3% in 13 evaluable patients, the RR was only 7.7%, with a median PFS of 3.1 months and a median OS of 6.2 months. Grade 3 hematological toxicities were seen in 23% of the patients, but no grade 4 toxicities were observed, and there were no treatment-related deaths.
Most patients with GBC are diagnosed at a late stage, in part, because there are few specific symptoms. Surgery is the only curative treatment. However, most patients are ineligible for surgery when diagnosed. The prognosis of patients with unresectable GBC is very poor, and most survive for less than a year after diagnosis [5]. During the 1980s and 1990s, a number of studies reported the effect of drugs, such as 5-fluorouracil (5 FU) or paclitaxel, on the management of patients who presented with locally advanced or metastatic biliary tract tumors, including GBC [6, 7]. However, these studies emphasized poor results in both the response and survival. Only after gemcitabine was used in the treatment of pancreatic cancer, and when it showed promising results, did it begin to be incorporated into GBC management. In one trial, 26 patients with unresectable GBC and no prior chemotherapy received single-agent gemcitabine. Of the 25 evaluable patients, an overall response rate of 36% (95% CI 17.1–57.9%) and a median survival time of 30 weeks was observed [8]. Thereafter, gemcitabine, in combination with other drugs, has been widely evaluated for patients with advanced biliary tract cancers (BTCs). The first major RCT to assess the efficacy and safety of GC in BTC was the phase II ABC-01 trial [9], which was extended into a phase III ABC-02 trial, the largest (n = 410) RCT in patients with BTC [3]. In the ABC-02 trial, GC significantly improved the OS, PFS, and tumor control rates (11.7, 8 months, and 81.4%, respectively) compared with the gemcitabine monotherapy (8.1, 5.0 months, and 71.8%, respectively). Based on the ABC-02 trial, GC has rapidly been accepted as the standard first-line treatment for advanced BTC. GC has also been accepted in Japan given a multicenter, randomized phase II study evaluating the efficacy and safety of GC compared with single-agent gemcitabine in 84 Japanese BTC patients [10]. The outcomes from this study were similar with the ABC-02 trial results, indicating a longer OS in the GC group compared to the single-agent group (11.2 vs. 7.7 months).
However, GBC has been reported to have a different biological behavior [4], and some evidence suggests that the efficacy of chemotherapy for GBC may differ from those of other primary sites in the biliary tract [11]. For instance, according to the recent systematic review of GC for advanced BTC [12], the study reporting both the lowest median OS and the highest response rate consisted exclusively of participants with GBC [4]. In this study that evaluated GC in 30 patients with unresectable GBC, the CR and PR were 13.3 and 23.3%, respectively, while the median OS was 20 weeks (95% CI 14–31 weeks) and 1-year survival rate was 18.6% [4].
Given these results, chemotherapy for GBC needs to be evaluated separately from other forms of BTC. Sharma et al. reported the only randomized control trial comparing best supportive care (BSC) with palliative chemotherapy for unresectable GBC in 82 patients. They reported that the RR of chemotherapy [modified gemcitabine plus oxaliplatin (mGEMOX)] was 30.8%, and the overall median survival in the mGEMOX arm was 9.5, whereas it was 4.5 months for the BSC arm (P value = 0.01) [13]. More recently, a non-randomized prospective study was reported evaluating the efficacy of chemotherapy (single agent and combination) over BSC in unresectable/metastatic GBC in a total of 85 patients [14]. The combination chemotherapy regimen was either 3 weekly gemcitabine–cisplatin (n = 45) or gemcitabine–oxaliplatin (n = 14) for a maximum of six cycles. The overall response rate to chemotherapy was 34%, including two patients with CR, with a median survival of the chemotherapy and BSC groups of 35.6 and 13 weeks, respectively (P value <0.001). The median OS values for the combination chemotherapy (n = 59) and the single-agent chemotherapy (n = 6) groups were 37 and 26.7 weeks, respectively (P value = 0.002). In our study, the RR was 7.7%, which was lower compared to these results. However, the above two studies were both published from India, and in the Indian subcontinent, the average age at diagnosis of GBC is approximately 50 years, which is significantly lower compared to the mean age of 65.2 years reported globally [14]. Thus, the characteristics of GBC, such as the age of onset, may vary by geographic region and racial–ethnic groups, and it might not be appropriate to interpret the results in different countries in the same way. Our result of a low RR could be related to the relative old age (mean 70 years old) and the higher incidence of metastatic lesions (84.6%).
Overall, the toxicity observed in this study was manageable. There was no grade 4 toxicity, and grade 3 toxicities were seen in only three patients, which were all hematological toxicities. It is to be noted that despite the higher incidence of hematological toxicity, drug-caused myelosuppression did not result in febrile neutropenia or bleeding.
Our study has several limitations. First, we did not perform a randomized trial comparing BSC and GC. The OS was less than 1 year, and the BSC might have shown similar results. However, the median survival of the untreated patients presenting with unresectable GBC is reported to be 2–4 months [2, 15], and our result of a median OS of 6.2 months is apparently superior to these results. Second, the number of patients included in this study was less than that of previous reports from India or those evaluating BTC patients. However, this is the first report evaluating the efficacy of a single regimen (GC) exclusively focused on chemo-naïve GBC patients without previous surgery in Japan.
In conclusion, gemcitabine in combination with cisplatin for unresectable GBC shows high DCR and relatively low toxicity, whereas the response rate was less than 10%, with a median survival of less than 1 year. The development of new therapies is desired to help improve the prognosis of this terrible cancer.
References
Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, La Vecchia C (2009) Epidemiology of biliary tract cancers: an update. Ann Oncol 20(1):146–159. doi:10.1093/annonc/mdn533
Gallardo J, Rubio B, Villanueva L, Barajas O (2005) Gallbladder cancer, a different disease that needs individual trials. J Clin Oncol 23(30):7753–7754. doi:10.1200/JCO.2005.02.7524 (author reply 7754–7755)
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABCT Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281. doi:10.1056/NEJMoa0908721
Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, Awasthy BS (2004) A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 90(8):1516–1520. doi:10.1038/sj.bjc.6601736
Leonard GD, O’Reilly EM (2005) Biliary tract cancers: current concepts and controversies. Expert Opin Pharmacother 6(2):211–223. doi:10.1517/14656566.6.2.211
Harvey JH, Smith FP, Schein PS (1984) 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol 2(11):1245–1248. doi:10.1200/JCO.1984.2.11.1245
Jones DV Jr, Lozano R, Hoque A, Markowitz A, Patt YZ (1996) Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol 14(8):2306–2310. doi:10.1200/JCO.1996.14.8.2306
Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C, Ahumada M (2001) A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 12(10):1403–1406
Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, Gollins S, Lofts F, Evans L, Meyer T, Anthoney A, Iveson T, Highley M, Osborne R, Bridgewater J (2009) Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study—The UK ABC-01 Study. Br J Cancer 101(4):621–627. doi:10.1038/sj.bjc.6605211
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, Koshiji M, Nambu Y, Furuse J, Miyazaki M, Nimura Y (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103(4):469–474. doi:10.1038/sj.bjc.6605779
Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, Sato T (2007) A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37(11):843–851. doi:10.1093/jjco/hym116
Park JO, Oh DY, Hsu C, Chen JS, Chen LT, Orlando M, Kim JS, Lim HY (2015) Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat 47(3):343–361. doi:10.4143/crt.2014.308
Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP (2010) Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 28(30):4581–4586. doi:10.1200/JCO.2010.29.3605
Singh SK, Talwar R, Kannan N, Tyagi AK, Jaiswal P, Kumar A (2016) Chemotherapy compared with best supportive care for metastatic/unresectable gallbladder cancer: a non-randomized prospective cohort study. Indian J Surg Oncol 7(1):25–31. doi:10.1007/s13193-015-0443-7
Perpetuo MD, Valdivieso M, Heilbrun LK, Nelson RS, Connor T, Bodey GP (1978) Natural history study of gallbladder cancer: a review of 36 years experience at M. D. Anderson Hospital and Tumor Institute. Cancer 42(1):330–335
Acknowledgements
We thank all the patients who participated in this study, their families, the investigators, and the study site personnel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relating to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hirooka, Y., Ishikawa, T., Kawashima, H. et al. Prospective multicenter phase II study of gemcitabine plus cisplatin in patients with unresectable gallbladder cancer. Cancer Chemother Pharmacol 80, 119–125 (2017). https://doi.org/10.1007/s00280-017-3341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3341-6