Abstract
Purpose
To determine a recommended dose for a biweekly combination neoadjuvant chemotherapy including gemcitabine, nab-paclitaxel, and S-1 (GAS) for patients with locally advanced pancreatic ductal adenocarcinoma (LAPC).
Methods
Patients with borderline resectable or unresectable LAPC without distant metastasis were eligible for this study. The planned dosages of gemcitabine (mg/m2, day 1), nab-paclitaxel (mg/m2, day 1), and S-1 (mg/day, days 1–7) were 800/100/60–100 at level 1, and 1000/125/60–100 at level 2. The treatment cycle was repeated every 2 weeks, and patients were assessed for resectability and response to the treatment after 6 cycles. This study was registered with UMIN Clinical Trial Registry (UMIN000016630).
Results
We enrolled 16 patients with LAPC in this study. At dose level 1, one of 8 patients experienced dose-limiting toxicity (DLT). One of the next 8 patients also experienced DLT at dose level 2. Based on these results, level 2 was considered the recommended dose for this regimen. Pancreatectomy with curative intent could be performed in 13 of the 16 patients. R0 resection was performed in 12 of 13 patients.
Conclusion
In conclusion, recommended doses for a biweekly GAS chemotherapy regimen were determined as nab-paclitaxel: 125 mg/m2, gemcitabine: 1000 mg/m2 on day 1, S-1: <1.25 m2, 60 mg; 1.25–1.5 m2, 80 mg; >1.5 m2, 100 mg twice a day on days 1–7. GAS chemotherapy showed good preliminary efficacy with mild toxicity in this study, and warrants a further phase 2 trial to investigate the efficacy of the GAS regimen for LAPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is one of the most lethal human cancers worldwide and is the fourth leading cause of cancer-related death in Japan and the USA [1, 2]. Overall 5-year survival rates for patients with pancreatic cancer are reported as 6% [3, 4]. The only way to cure patients with pancreatic cancer is complete surgical resection. In addition, a survival benefit of adjuvant chemotherapy has been proven in large-scale randomized-controlled trials [5, 6]. However, more than 70% of patients are not candidates for these standard therapeutic strategies for resectable pancreatic cancer because they have locally advanced or metastatic pancreatic cancer at the time of diagnosis [7]. Of these patients, about 40% suffer from locally advanced, but nonmetastatic PDAC [8]. Standard therapy for these patients remains controversial, whereas chemotherapy is recognized as standard therapy for patients with distant metastatic pancreatic cancer.
To increase the number of patients with locally advanced pancreatic cancer (LAPC) who can be offered a chance of cure after radical pancreatectomy, efforts are being made to develop a more effective preoperative therapeutic strategy including chemo- and chemoradiotherapy for LAPC [9–13]. Recently, the MPACT trial found that nab-paclitaxel combined with gemcitabine prolonged the median survival time (MST) from 6.4 to 8.5 months over gemcitabine monotherapy in patients with metastatic pancreatic cancer [14]. By contrast, subanalysis in the GEST study, a randomized phase 3 study from Japan, revealed that the MST of patients with LAPC treated with gemcitabine+S-1 combination therapy was 15.9 months [15]. Moreover, a meta-analysis revealed that gemcitabine+S-1 improved objective response rate and survival over gemcitabine monotherapy in patients with LAPC [16]. Thus, gemcitabine, nab-paclitaxel, and S-1 are good candidates for LAPC treatment. Based on these findings, a triple chemotherapeutic regimen including gemcitabine, nab-paclitaxel, and S-1 was designed for a neoadjuvant setting expecting a promising efficacy for LAPC. The aim of this phase 1 study was to determine a recommended dose for a biweekly combination neoadjuvant chemotherapy including gemcitabine, nab-paclitaxel, and S-1 (GAS) for patients with LAPC.
Patients and methods
Patients and eligibility
Patients eligible for study entry had LAPC which was classified into borderline resectable or unresectable pancreatic cancer without distant metastasis based on the resectability status of the National Comprehensive Cancer Network (NCCN) 2016, version 2 [17]. Eligibility criteria were as follows: (1) a histologically or cytologically proven diagnosis of adenocarcinoma or adenosquamous carcinoma; (2) tumor with contact with the major arteries including the superior mesenteric artery, celiac artery, or common hepatic artery on pretreatment computed tomography (CT); (3) no previous antitumor treatment except for biliary drainage; (4) age between 20 and 79; (5) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (6) adequate hematological, hepatic, and renal functions by hemoglobin ≥8.0 g/dL, leucocytes ≥3000/mm3 and ≤12,000/mm3, neutrophils ≥1500/mm3, platelets ≥100,000/mm3, total bilirubin ≤2.0 mg/dL, aspartate aminotransferase and alanine aminotransferase ≤100 U/L, serum creatinine ≤1.2 mg/dL, and albumin ≤3.0 g/dL; and (7) adequate oral intake.
This phase 1 study was conducted in compliance with the ethical principles of the Declaration of Helsinki, and the protocol was approved by the institutional review board at each participating institution. All patients provided written informed consent before their enrollment in the study. This clinical study was registered in the University Medical Information Network–Clinical Trial Registry (UMIN-CTR), identification number 000016630.
Treatment and efficacy assessment
This study was an open label, single arm, phase I study for patients with LAPC conducted at three institutions in Hiroshima. The primary endpoints were to evaluate the frequency of dose-limiting toxicities (DLTs) and to determine recommended doses for the GAS chemotherapy regimen. The secondary endpoints were to evaluate the efficacy of the regimen for LAPC. Patients were assigned to two levels of GAS regimens. Nab-paclitaxel was administered intravenously at 100 mg/m2 over 30 min in level 1, and 125 mg/m2 in level 2, followed by intravenous gemcitabine at 800 mg/m2 over 30 min on day 1 in level 1, and 1000 mg/m2 in level 2. A daily dose of S-1 in both levels was defined based on the patient’s body surface area as follows: <1.25 m2, 60 mg; 1.25–1.5 m2, 80 mg; >1.5 m2, 100 mg. S-1 dose was fixed in both levels and administered orally twice a day on days 1–7. The treatment cycle was repeated every 2 weeks. After 6 cycles of the GAS chemotherapy regimen, patients were reevaluated to assess resectability and response to the treatment. Tumor response was evaluated using multidetector CT after completion of a 6 cycle GAS regimen according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [18, 19]. If deemed resectable for cure, patients underwent surgical exploration, whereas those in whom the PDAC progressed to being unresectable received additional chemotherapy. Tumor stage, lymph node metastasis, and final stage were classified based on the 7th edition of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TMN) classification [20, 21].
Dose escalation and definition of dose-limiting toxicities
DLTs were determined during the 6 cycles of neoadjuvant chemotherapy. DLT was defined based on the common toxicity criteria adverse event (CTCAE) version 4.0, as one or more of the following events: (1) grades 3–4 of neutropenia complicated by fever, (2) grade 4 of leucopenia or neutropenia, (3) grades 3–4 of anemia or thrombocytopenia, (4) grades 3–4 of nonhematological toxicities, and (5) more than 2 weeks of drug withdrawal during the first cycle. Eight patients were enrolled for each dose level. If DLTs occurred in three of the initial eight patients enrolled in level 1, this phase I study was to be discontinued as an intolerable regimen. If DLTs were observed in 2 or fewer patients among the initial eight enrolled in level 1, the next eight patients were enrolled in level 2. If DLTs were observed in two or fewer patients among the eight enrolled in level 2, level 2 was to be defined as the recommended dose. If DLTs were observed in three patients in level 2, level 1 was to be defined as the recommended dose. The protocol chemotherapy was started and repeated on day 1 when the neutrophils ≥1000 /mm3, platelets ≥75,000 /mm3, serum creatinine ≤1.2 mg/dL, no stomatitis or diarrhea of grade 2 or higher. An additional course was delayed until recovery if a patient’s condition did not meet the eligibility criteria described above. Moreover, in the case of more than 21 days of postponement, this protocol treatment was discontinued.
Results
Patient characteristics
Sixteen patients with LAPC were enrolled in this study between March 2015 and June 2016. Characteristics of these 16 patients are summarized in Table 1. Of the 16 patients, nine were male and seven were female with a median age of 67 years (range, 51–78). The median of maximum tumor size at registration was 39 mm (range, 20–73). According to the resectability status of NCCN 2016 version 2, 14 patients were diagnosed as having borderline resectable pancreas cancer, whereas two were diagnosed as having unresectable LAPC. Of the two patients with unresectable LAPC, one had a tumor contacting the common hepatic artery with extension to the hepatic artery bifurcation, and the other had a tumor contacted with superior mesenteric artery more than 180°.
Toxicity
Occurrence of DLTs is listed in Table 2. At dose level 1, one of eight patients experienced DLT: grade 4 neutropenia that recovered within a few days and did not require suspension of the treatment protocol. Therefore, the next eight patients were enrolled in level 2. As in level 1, one patient experienced a DLT: grade 4 neutropenia for a short time that did not require suspension of the treatment protocol. Based on these results, level 2 was considered as a recommended dose for this regimen. Details of toxicities observed in the 16 enrolled patients during the 6-cycle course are listed in Table 3. Two patients experienced grade 3 leukopenia (level 1: n = 1, level 2: n = 1) and 3 experienced grades 3–4 neutropenia (level 1: n = 1, level 2: n = 2). Nonhematological toxicity included only grade 3 cholangitis observed in only one patient at the level 1 dosage. There was no occurrence of other grade 3–4 nonhematological toxicity or treatment-related death at either level. No patient needed dosage reduction or required more than 2 weeks of drug withdrawal during any cycle.
Treatment response
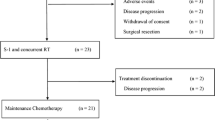
All patients were included in the response evaluation, and the possibility of surgical resection was explored. An outline of the overall response to protocol treatment including surgical resection is summarized in Table 4. No patient had a complete response as determined by RECIST version 1.1. Partial response was achieved in five patients, and ten patients had stable disease. Progression of the disease occurred in one patient with initially borderline resectable pancreatic cancer, which contacted the celiac artery at more than 180°. Peritoneal metastasis was found in this patient by CT imaging when 5 cycles of GAS chemotherapy had been completed. Therefore, resectability status of this patient was changed from borderline resectable to unresectable after the treatment protocol. By contrast, resectability status was not changed from pre- to post-GAS chemotherapy in the other 15 patients. Pancreatectomy with curative intent was performed in 13 patients. The other three patients did not undergo surgical resection: one patient had a distant metastasis; one was not a surgical candidate because of severe liver cirrhosis at the time of surgical evaluation; and one refused surgical resection. Median reduction rates of CA19-9 and DUPAN-II from baseline to post-GAS chemotherapy were 75.0 and 40.5%, respectively.
Surgical outcomes
Of the 13 patients who underwent surgical resection, 12 had borderline resectable PC and one had unresectable PC. Surgical procedures included pylorus-preserving pancreatoduodenectomy (PPPD) with portal or superior mesenteric vein resection in six patients, distal pancreatectomy (DP) in 2, and DP with celiac artery resection (DP-CAR) [22] in 4. One patient who had unresectable LAPC underwent PPPD with hepatic artery resection and reconstruction using arterial graft interposition. Pathological examination of resected specimens demonstrated that 12 patients had T3 tumors, and one had a T4 tumor. Eleven patients had lymph node metastases. Twelve patients underwent R0 resection. Histological response evaluation based on the Evans classification [23] revealed five patients with grade I, four with grade IIA, three with grade IIB, and one with Grade III.
Discussion
This phase 1 study evaluated the safety and dose for a biweekly schedule of GAS chemotherapy, a triple regimen of gemcitabine, nab-paclitaxel, and S-1, for patients with LAPC. A high response rate without severe toxicity is required for an ideal preoperative therapeutic regimen for LAPC. A marked shrinking of tumor size can increase the chance of curative resection for LAPC. By contrast, the physical condition of patients with LAPC who receive preoperative therapy should be maintained during the treatment protocol to enable tolerance of subsequent major pancreatectomy for LAPC, such as PPPD with major vessel resection or DP-CAR. Considering the remarkable efficacy of the GEST Study and MPACT trial [14, 15], a triple regimen including gemcitabine, nab-paclitaxel, and S-1 is attractive for LAPC. However, toxicities making this treatment unacceptable are also of concern if these drugs are simultaneously administered at the same dose as used in the previous trials. Actually, a recent phase I / II study of gemcitabine plus nab-paclitaxel (125 mg/m2 of nab-p plus 1000 mg/m2 of gemcitabine on day 1, 8, 15 every 4 weeks) for Japanese patients with metastatic pancreatic cancer revealed that 85.3% of patients have experienced grade 3 or higher toxicity [24]. Therefore, a biweekly schedule was adopted to minimize the severe adverse events in this study, while keeping the dose at each administration identical to that in standard therapy.
In the current study, DLT was observed in only two patients (one at each level). Both of these patients experienced grade 4 neutropenia. The first patient enrolled in level 1 suffered from grade 3 cholangitis because of obstruction of biliary drainage at cycle 2, which occurred just before the appearance of grade 4 neutropenia. This cholangitis probably worsened the neutropenia. This patient recovered quickly after exchange of biliary drainage. The second patient enrolled in level 2 experienced grade 4 neutropenia at cycle 1, and quickly recovered without specific treatment. Grade 3 leukopenia was observed in these patients at the same time as their grade 4 neutropenia. No patient experienced febrile neutropenia. Consequently, level 2 (nab-paclitaxel: 125 mg/m2, gemcitabine: 1000 mg/m2 on day 1, S-1: <1.25 m2, 60 mg; 1.25–1.5 m2, 80 mg; >1.5 m2, 100 mg twice a day on days 1–7) was selected as the recommended dose for a subsequent phase 2 study. Only one severe adverse event related to nonhematological toxicity (grade 3 of cholangitis in one patient) occurred during the treatment protocol. Notably, grades 3–4 of peripheral neuropathy, which is a common adverse event during use of nab-paclitaxel, was not observed in any enrolled patient. Severe peripheral neuropathy frequently detracts from a patients’ quality of life and forces reduction or discontinuation of nab-paclitaxel. The overall rate of adverse events during levels 1 and 2 of the GAS chemotherapy was 19%, which was less than those reported in previous clinical studies of gemcitabine and nab-paclitaxel, or gemcitabine and S-1. This mild toxicity of the GAS regimen is favorable because it maintains a heathy physical condition of the patients for subsequent major pancreatectomy.
Although assessment of efficacy was not the main objective of this phase 1 study, the GAS chemotherapy regimen showed a favorable anticancer activity. This regimen showed a response in 31% and disease control in 94%, which was comparable to those of FOLFIRINOX or gemcitabine plus S-1 in patients with unresectable LAPC [16, 25]. Pancreatectomy with curative intent could be performed in 12 of 14 patients with initially borderline resectable PDAC and 1 of the two patients with initially unresectable PDAC. Moreover, curative resection (R0) was achieved in 12 of 13 patients who underwent surgical resection. This R0 resection rate was comparable to or higher than that of previous neoadjuvant trials based on chemoradiation therapy, in which the R0 resection rate was 65–100% [12, 26–28]. By contrast, interest in application of FOLFILINOX as a neoadjuvant has been increasing since its high response rate and disease control rate for metastatic pancreatic cancer were demonstrated [29]. However, concern remains that patients treated with FOLFILINOX may drop out and lose the chance of surgical resection because of the severe toxicity of this treatment. Indeed, a review of neoadjuvant FOLFILINOX for borderline or unresectable pancreatic cancer by Petrelli et al. [30] reported the cumulative rate of grades 3–4 toxicity ranging from 28.7 to 75% in 9 studies. Compared with FOLFILINOX, the toxicity of the GAS regimen appeared to be mild. Because both the efficacy and toxicity profile of the GAS regimen were attractive, a phase 2 trial is warranted to clarify the anticancer activity, toxicity, and prognostic impact of the GAS level 2 regimen for LAPC.
In conclusion, recommended doses for a biweekly GAS chemotherapy regimen were determined as nab-paclitaxel: 125 mg/m2, gemcitabine: 1000 mg/m2 on day 1, S-1: <1.25 m2, 60 mg; 1.25–1.5 m2, 80 mg; >1.5 m2, 100 mg twice a day on days 1–7. GAS chemotherapy showed good preliminary efficacy with mild toxicity in the present study. These findings warrant a phase 2 trial, which is now underway in multiple institutions, to investigate further the efficacy of GAS chemotherapy for LAPC.
References
Ministry of Health, Labour and Welfare The Dynamic Statistics of the Population in 2015. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei15/index.html. Accessed 1 Nov 2016
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. The Lancet 363:1049–1057
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Oettle H, Post S, Neuhaus P et al (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. Jama 297:267–277
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). The Lancet 388:248–257
Matsuno S, Egawa S, Fukuyama S et al (2004) Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas 28:219–230
Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7:163–172
Sherman WH, Chu K, Chabot J et al (2015) Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer 121:673–680
Sahora K, Kuehrer I, Eisenhut A et al (2011) NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 149:311–320
Motoi F, Ishida K, Fujishima F et al (2013) Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol 20:3794–3801
Reni M, Cereda S, Balzano G et al (2009) Outcome of upfront combination chemotherapy followed by chemoradiation for locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 64:1253–1259
Murakami Y, Uemura K, Sudo T et al (2016) Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol 79(1):37–47
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648
Yanagimoto H, Ishii H, Nakai Y et al (2014) Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci 21:761–766
National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN Guidelines). (2016). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 1 Nov 2016
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Padhani AR, Ollivier L (2001) The RECIST (response evaluation criteria in solid tumors) criteria: implications for diagnostic radiologists. Br J Radiol 74:983–986
James D., Brierley MKG, Christian Wittekind (2009) TNM Classification of Malignant Tumours, 7th Edition. Wiley-Blackwell, New York
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Hirano S, Kondo S, Hara T et al (2007) Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 246:46–51
Evans DB, Rich TA, Byrd DR et al (1992) Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127:1335–1339
Ueno H, Ikeda M, Ueno M et al (2016) Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 77:595–603
Suker M, Beumer BR, Sadot E et al (2016) FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 17:801–810
Allendorf JD, Lauerman M, Bill A et al (2008) Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg 12:91–100
Golcher H, Brunner T, Grabenbauer G et al (2008) Preoperative chemoradiation in adenocarcinoma of the pancreas. A single centre experience advocating a new treatment strategy. Eur J Surg Oncol 34:756–764
Wilkowski R, Thoma M, Bruns C et al (2006) Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP 7:349–360
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Petrelli F, Coinu A, Borgonovo K et al (2015) FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 44:515–521
Funding
This study was never funded by any foundation, company, industry, or external source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any commercial interests associated with this study.
Disclosure of commercial interest
None of the authors has any commercial interests associated with this study or received any financial or material support for this study.
Ethical approval
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and the protocol was approved by the institutional review board at each participating institution. All patients provided written informed consent before their enrollment in the study.
Rights and permissions
About this article
Cite this article
Kondo, N., Murakami, Y., Uemura, K. et al. A phase 1 study of gemcitabine/nab-paclitaxel/S-1 (GAS) combination neoadjuvant chemotherapy for patients with locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 79, 775–781 (2017). https://doi.org/10.1007/s00280-017-3274-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3274-0