Abstract
Purpose
Anti-epidermal growth factor receptor antibody therapy alone or in combination with irinotecan is recognized as a standard third-line treatment for KRAS wild-type unresectable metastatic colorectal cancer. However, in some cases, it is difficult to administer irinotecan after third-line treatment. Therefore, we examined the efficacy and safety of the combination of cetuximab and S-1 in patients with KRAS wild-type unresectable metastatic colorectal cancer who were previously treated with irinotecan, oxaliplatin, and fluoropyrimidines.
Methods
The study was designed as a phase II, non-randomized, open-label, multicenter trial. Cetuximab was initially administered at 400 mg/m2, followed by weekly infusion at 250 mg/m2. S-1 was administered at a fixed dose of 80 mg/m2 orally twice daily for 28 days followed by a 14-day break, resulting in a 6-week treatment course. The primary endpoint was progression-free survival (PFS). The secondary endpoints were the overall response rate (ORR), overall survival (OS), disease control rate (DCR), time to treatment failure, dose intensity, safety, and BRAF mutation status.
Results
Thirty-seven patients were eligible. The median PFS was 5.5 months, the median OS was 13.5 months, the ORR was 29.7 %, and the DCR was 73.0 %. The relative dose intensity was 86.8 % for cetuximab and 88.1 % for S-1. Grade 3–4 adverse events that occurred in >10 % of the patient population included rash, dry skin, diarrhea, paronychia, anorexia, fatigue, mucositis, and neutropenia.
Conclusions
Combination therapy with cetuximab and S-1 was effective and well tolerated in patients with irinotecan-, oxaliplatin-, and fluoropyrimidine-refractory metastatic colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in chemotherapy over the last decade have helped increase the median OS of patients with metastatic colorectal cancer, and recent studies illustrated that the proportion of patients surviving for more than 2 years has increased. These advances have mainly been attributable to the introduction of irinotecan and oxaliplatin. Additionally, the emergence of molecularly targeted drugs is considered another factor that has further extended patient survival [1].
The epidermal growth factor receptor (EGFR) signaling pathway regulates cell differentiation, proliferation, migration, angiogenesis, and apoptosis, all of which become deregulated and activated in cancer cells. Cetuximab (Cmab) is a chimeric IgG1 monoclonal antibody that binds to and blocks EGFR with high specificity [2, 3].
The molecularly targeted drugs available for unresectable metastatic colorectal cancer (mCRC) include the anti-EGFR antibodies Cmab and panitumumab (Pmab), the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab (Bmab), and the multi-kinase inhibitor regorafenib, which recently became available in Japan. Cmab treatment achieved significantly superior OS, PFS, and ORR compared to best supportive care (BSC) in the CO.17 study, a phase III randomized control study that compared third-line Cmab monotherapy to BSC [4]. Third-line Cmab plus irinotecan achieved a superior ORR and PFS compared to Cmab monotherapy in a phase II trial (BOND study) in irinotecan-refractory patients [5]. As a result of these 2 clinical trials, Cmab monotherapy and Cmab plus irinotecan combination therapy are recognized as standard third-line therapies in clinical settings.
Mutations in KRAS, which encodes a protein downstream from EGFR, have been widely noted as predictors of Cmab efficacy. A published analysis that was stratified on the basis of the presence of KRAS mutations revealed that Cmab provided an additional effect in patients with KRAS wild-type (WT) tumors but not in those with KRAS mutant (MUT) tumors [6–8].
It has been reported that anti-EGFR antibodies, including Cmab, have increased potency in combination with chemotherapy and radiotherapy compared to treatment with any of these agents alone [2, 3]. In the BOND study, the group treated with Cmab plus irinotecan experienced a better anti-tumor effect than the group treated with Cmab alone [5]. There are no clinical data for the combination of Cmab and S-1, but there are fundamental data for the combination of Cmab and 5-FU/S-1. Previous basic research studies using colorectal, pancreatic, and gastric cancer cells found that the combination of Cmab and 5-FU had an enhanced anti-tumor effect compared to treatment with either drug alone [9–12]. S-1 is an oral anticancer agent that contains tegafur (a 5-FU prodrug) and 2 modulators: gimeracil and oteracil potassium. Gimeracil inhibits dihydropyrimidine dehydrogenase (DPD), an enzyme that degrades 5-FU, thereby supporting the maintenance of high levels of 5-FU in the blood. S-1 exerts a strong anti-tumor effect, and the drug can be expected to be effective in patients with 5-FU-resistant tumors as a result of high DPD levels in their tumors [13].
For this reason, we hypothesized that combination therapy with Cmab and S-1 would have an enhanced effect in patients with 5-FU-resistant tumors. Therefore, we initiated a phase II clinical study to investigate the efficacy and safety of Cmab plus S-1 combination therapy in patients with colorectal cancer who were previously treated with fluoropyrimidines, irinotecan, and oxaliplatin.
Materials and methods
Patient eligibility
This study was designed as a phase II, non-randomized, open-label, multicenter trial. This trial was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000002475). To be eligible, patients had to have histologically proven colorectal cancer, clinically proven unresectable mCRC, and a measurable lesion judged by contrast-enhanced computed tomography (CT) [according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST)]. Eligible patients also had a history of prior treatment with irinotecan, oxaliplatin, and fluoropyrimidines, documented progressive disease (PD) on 5-FU-based chemotherapy, an Eastern Cooperative Oncology Group performance status (PS) of 0 or 1, an age of more than 20 years and less than 80 years, and sufficient bone marrow, liver, and kidney function. EGFR expression in primary or metastatic tumor tissue was confirmed by immunohistochemical evaluation. KRAS WT status in codons 12 and 13 was confirmed by the KSCC central test. The study was conducted in accordance with ethical principles underlying in the Declaration of Helsinki. All patients signed informed consent forms. The institutional ethics committees of all participating centers approved this study.
Treatment
Cmab was administered initially at a dose of 400 mg/m2 followed by weekly infusion at 250 mg/m2. S-1 was administered at a fixed dose of 80 mg/m2 orally twice per day for 28 days followed by a 14-day break, resulting in a 6-week treatment course.
Evaluation of patients
Physical examinations, laboratory testing, and safety assessments were performed once before the start of treatment and weekly thereafter. Tumor response was evaluated every 6 weeks by contrast-enhanced CT. The assessment was performed by the investigators using RECIST. Adverse events were assessed according to the National Cancer Institute Common Toxicity Criteria version 3.0. The Cmab dose was only modified in patients who experienced toxic skin effects. Treatment was immediately discontinued in patients who exhibited a grade 3 or higher hypersensitivity reaction, and the dose of S-1 was modified in patients who experienced hematologic or non-hematologic toxic effects.
Statistical analysis
The primary endpoint was PFS. The secondary endpoints were the ORR, OS, DCR, TTF, dose intensity, safety, and BRAF mutation. The study was designed to detect an improvement in the median PFS (mPFS) from 2.5 to 3.5 months based on the previous experience with Cmab monotherapy. Assuming a type I error rate of 5 and 80 % power, 37 patients were required.
The PFS, OS, and TTF curves were calculated using the Kaplan–Meier method. The 90 % confidence interval (CI) for PFS and 95 % CIs for other survival measures was estimated using Brookmeyer and Crowley method. To analyze the anti-tumor effect as secondary endpoints, the response rate was calculated, and the 95 % CI was estimated using the Clopper-Pearson exact method based on the binominal distribution. All statistical analyses were performed using SAS for Windows release 9.3 (SAS Institute, Cary, NC, USA).
BRAF mutation
BRAF gene analysis was performed via a direct sequencing method and a pyro-sequencing method using DNA samples extracted from cancer cells separated from a slice of a paraffin block by micro-dissection.
Results
Patient characteristics
Fifty-nine patients were registered during the primary registration period from October 2009 to December 2010 at 27 institutes. In this protocol, KRAS gene analysis and EGFR immunostaining were performed by central management. As a result of KRAS genetic testing, secondary registration was not possible for patients with KRAS mutations. KRAS gene testing was performed on DNA samples extracted from cancer cells separated from slices of paraffin blocks using a micro-dissection method. In total, 20 patients were not included in the secondary registration. The reasons for exclusion were KRAS mutation (12 patients), EGFR negativity (3 patients), abnormal laboratory value (3 patients), no informed consent (1 patient), and doctor judgment (1 patient). During secondary registration, 39 patients were enrolled in the study. However, 2 patients were ineligible for study inclusion because of the doctor’s misinterpretation of the eligibility criteria (1 patient) and because a KRAS mutation was found in the central investigation in spite of a KRAS WT finding in the institutional investigation (1 patient), resulting in 37 eligible patients. The consort diagram for this study is shown in Fig. 1.
The patient characteristics are summarized in Table 1. The PS was 0 in 32 patients and 1 in 5 patients. There were 15 patients (40.5 %) with metastasis to 1 organ, 15 patients (40.5 %) with metastasis to 2 organs, and 7 patients (18.9 %) with metastasis to 3 or more organs. Concerning the number of previous treatment regimens, 4 patients (10.8 %) had received first-line treatment, 23 patients (62.2 %) had received second-line treatment, 7 patients (18.9 %) had received third-line treatment, and 3 patients (8.1 %) had received fourth-line or more treatment. All four patients receiving only one previous chemotherapy regimen were treated with an alternative sequential therapy of mFOLFOX6 and FOLFIRI (FIREFOX) with registration of the clinical trial.
Efficacy
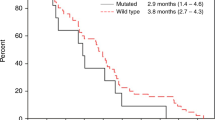
The mPFS, the primary endpoint, was 5.5 months (90 % CI 4.4–5.7 months) (Fig. 2). As the lower limit of the 2-sided 90 % CI of the observed mPFS was greater than 3.5 months, this regimen was judged to be effective. The confirmed ORR was 29.7 % (11/37 patients; 95 % CI 15.9–47.0 %) for all eligible cases (complete response [CR], 1 patient; partial response [PR], 10 patients; stable disease, 16 patients; PD, 8 patients; not evaluable, 2 patients). The DCR was 73.0 % (27/37 patients). The median OS (mOS) was 13.5 months (95 % CI 8.5–16.5 months; Fig. 3). The TTF was 4.6 months (95 % CI 3.2–5.6 months).
Duration of treatment and the relative dose intensity of Cmab and S-1
The median duration of treatment was 4 courses (range, 1–12 courses). The relative dose intensity was 86.8 % for Cmab and 88.1 % for S-1. Dose intensity was calculated as the actual dose per week divided by the planned dose per week.
Adverse events
Table 2 lists the numbers of patients who experienced adverse events classified by symptom for all courses. Grade 3 or higher hematologic adverse events included neutropenia in 4 patients (10.8 %), increased blood bilirubin in 3 patients (8.1 %), hypomagnesemia in 2 patients (5.4 %), anemia in 2 patients (5.4 %), and elevated alanine transaminase, elevated aspartate transaminase, leukocytopenia, and thrombocytopenia in 1 patient (2.7 %) each. Grade 3 or higher non-hematologic adverse events included rash in 10 patients (27.0 %), dry skin in 5 patients (13.5 %), anorexia in 4 patients (10.8 %), diarrhea in 4 patients (10.8 %), mucositis in 4 patients (10.8 %), paronychia in 4 patients (10.8 %), fatigue in 4 patients (10.8 %), pruritus in 3 patients (8.1 %), and nausea and vomiting in 1 patient (2.7 %) each. No serious adverse events were particularly observed.
Post-protocol treatment
Post-protocol treatment occurred in 18 of 37 eligible patients (48.6 %). Nine patients received 1 regimen, 4 patients received 2 regimens, and 5 patients received 3 or more regimens. Concerning the regimen administered immediately after protocol completion, 6 patients received an irinotecan-based regimen, 2 patients received an oxaliplatin-based regimen, 6 patients received fluoropyrimidines, and 4 patients received molecularly targeted monotherapy.
BRAF mutation
BRAF was not analyzed in 1 of the 37 patients. BRAF was WT in 34 patients, and only 2 patients (5.4 %) had BRAF MUT.
Discussion
Cmab inhibits EGFR, and it has been demonstrated to exert an anti-tumor effect by suppressing the activation of MAPK and AKT, which are downstream factors in the EGFR pathway, and by inhibiting proliferation, infiltration, and angiogenesis in cancer cells [2, 3]. An association between KRAS and Cmab efficacy was reported in a study of anti-EGFR antibodies such as Cmab and Pmab [6–8, 14]. This current KSCC 0901 study was a phase II clinical trial investigating the efficacy and safety of Cmab plus S-1 combination therapy as a third-line treatment in patients with EGFR-positive, KRAS WT, unresectable mCRC. The patients in this study had also been previously treated with 3 chemotherapy agents (fluoropyrimidines, oxaliplatin, and irinotecan). The mPFS was 5.5 months for patients treated with Cmab plus S-1. The mPFS of the combination therapy exceeded 3.5 months, which was the predicted mPFS for Cmab monotherapy, and thus, the effectiveness of this regimen was confirmed. In addition, the confirmed ORR and DCR were good (29.7 and 73.0 %, respectively). The best ORR was 37.8 % (1 patient with CR, 13 patients with PRs), and the DCR was 97.3 % (not confirmed). The mOS was 13.5 months. The median relative dose intensity was good: 86.8 % for Cmab and 88.1 % for S-1. Furthermore, although 89.2 % of the registered patients had received third-line or further treatment, post-protocol treatment was administered to nearly half of the patients (18/37 patients: 48.6 %). This indicates that Cmab plus S-1 combination therapy is a highly tolerable and safe treatment. This is the first prospective study to demonstrate that combination therapy with Cmab plus S-1 is effective and well tolerated as a third-line treatment for mCRC.
The results from phase II and phase III clinical trials of anti-EGFR antibodies as third-line treatments in patients with KRAS WT mCRC are shown in Table 3. According to this sub-analysis, in the phase III CO.17 study, the mPFS was 3.7 months for Cmab monotherapy, versus 1.9 months for BSC in patients with KRAS WT mCRC (hazard ratio [HR] 0.40; p < 0.001). The mOS was 9.5 months for the Cmab group, compared to 4.8 months for the BSC group (HR 0.55; p < 0.001). However, in KRAS MUT patients treated with Cmab monotherapy, the mPFS was 1.8 versus 1.8 months for BSC, and the mOS was 4.5 versus 4.6 months for BSC. There was no difference between the Cmab monotherapy and BSC groups in KRAS MUT patients [8]. The results obtained using another anti-EGFR antibody, Pmab, were similar to those observed for Cmab. A phase III randomized controlled study, the 20020408 study, demonstrated the efficacy of Pmab compared to BSC as a third-line treatment in chemotherapy-intolerant patients with mCRC [14, 15]. A sub-analysis of patients treated with Pmab monotherapy or BSC found that the mPFS in patients with KRAS WT mCRC were 12.3 weeks (3.1 months) and 7.3 weeks (1.8 months), respectively (HR, 0.45; p < 0.0001). The mPFS in the KRAS WT mCRC group was significantly longer than that of the KRAS MUT group (p < 0.0001) [14]. In the current study (KSCC 0901), the Cmab plus S-1 combination therapy group had a mPFS of 5.5 months, a mOS of 13.5 months, and an ORR of 29.7 % for patients with KRAS WT mCRC. These results were clearly better than those obtained using Cmab monotherapy for patients with KRAS WT mCRC in the CO.17 study and those obtained for Pmab monotherapy for patients with KRAS WT mCRC in the 20020408 study. A single-arm phase II trial (the PIMABI study) was performed to investigate the efficacy and safety of Pmab plus irinotecan as a third-line treatment for KRAS WT mCRC. In the PIMABI study, the ORR was 29.2 %, the mPFS was 5.5 months, and the mOS was 9.7 months [16]. In addition, Shitara et al. [17, 18] reported two results of a phase II trial of Cmab (weekly) plus irinotecan and Cmab (biweekly) plus irinotecan as a third-line treatment for KRAS WT mCRC. The ORRs were 30.0 and 30.0 %, the mPFSs were 5.8 and 5.3 months, and the mOSs were not reached and 10.8 months in patients receiving Cmab (weekly) plus irinotecan and Cmab (biweekly) plus irinotecan, respectively. These results are similar to those in this study, and the Cmab plus S-1 and Cmab/Pmab plus irinotecan regimens are considered equally effective. The BOND study examined the effectiveness of Cmab plus irinotecan combination therapy versus Cmab monotherapy as third-line treatments in irinotecan-resistant patients; however, the reported results were not stratified by KRAS status [5]. Therefore, these results cannot be compared to Cmab plus S-1 combination therapy in patients with KRAS WT mCRC. The results of a phase II clinical trial of the Cmab plus irinotecan combination as a third-line treatment for irinotecan-, oxaliplatin-, and 5-FU–refractory mCRC were reported in Japan. The ORR, DCR, TTF, and mOS were 30.8, 64.1 %, 4.1, and 8.8 months, respectively [19]. In a Japanese phase II clinical trial of Pmab monotherapy, the response rate, mPFS, and mOS were 13.5 %, 8.0 weeks (2 months), and 9.3 months, respectively [20]. It is likely that these results would have illustrated better efficacy if the subjects were limited to only KRAS WT mCRC.
Serious adverse events were not observed. Grade 3 or higher adverse events that were observed in at least 10 % of patients-included neutropenia (10.8 %) as a hematopoietic toxicity and rash (27.0 %), dry skin (13.5 %), paronychia (10.8 %), anorexia (10.8 %), diarrhea (10.8 %), fatigue (10.8 %), and mucositis (10.8 %) as non-hematopoietic toxicities. The adverse events observed in this study were compared to those recorded in the BOND study, the EPIC study, and a domestic phase II/III study of Cmab plus irinotecan combination therapy. Grade 3 or higher adverse events such as neutropenia, leukopenia, anemia, and diarrhea tended to be rare, whereas rash occurred more frequently in this study [5, 17–19, 21]. Therefore, there are few serious adverse events, and the regimen is believed to be well tolerated.
The patients who participated in our clinical trial had a good PS in comparison with other clinical trials (Table 3). As a result of their good PS, the post-protocol treatment was administered in a high proportion of patients. Thus, we cannot deny that the outcomes of our Cmab+S-1 regimen may have been affected by this. However, there are no clinical trials of the combination therapy of Cmab and S-1 as any line of chemotherapy, but there are two clinical trial reports that show the safety and effectiveness of the combination of oxaliplatin/S-1 (SOX)+cetuximab as first-line chemotherapy for KRAS WT mCRC in Japan [22, 23]. One of those was a report from our group (KSCC1002). The overall response was 63.6 %, and the rate of R0 liver resection was 39.4 % for colorectal cancer patients with initially unresectable or not optimally resectable liver metastases [22]. S-1 and oxaliplatin plus cetuximab as a first-line treatment had promising results. In addition, there are some basic research studies that indicate the advantage of the combination of S-1 and Cmab [9–12]. Thus, we consider that combination therapy with S-1 and Cmab probably produces a promising result. The effectiveness of S-1+Cmab combination therapy as third-line chemotherapy will become clearer following the randomized phase III clinical trial of Cmab with or without S-1 in future.
S-1 is an oral anticancer agent widely used in the treatment of various cancers, including colorectal cancer, in Japan. However, some differences in the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients have been reported [24]. S-1 could potentially cause a high incidence of gastrointestinal toxicity in Caucasian patients with the dose used in this study. These findings should be considered in daily practice.
The results of the current study confirmed the efficacy and safety of combination therapy with Cmab and S-1 as a third-line treatment in patients with KRAS WT mCRC who had previously been treated with 3 drugs (fluoropyrimidines, irinotecan, and oxaliplatin). It is difficult to administer irinotecan to some patients owing to its adverse events. For example, irinotecan is associated with adverse events in patients at high risk for irinotecan-induced toxicity because of polymorphisms in the UDP-glucuronosyltransferase 1 family, polypeptide A1 gene. We believe that the Cmab plus S-1 regimen examined in this study will become an optional third-line treatment regimen.
References
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 27:3677–3683
Baselga J (2001) The EGFR as a target for anticancer therapy-focus on cetuximab. Eur J Cancer 37:S16–S22
Ciardiello F, Tortora G (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958–2970
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras Mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Nukatsuka M, Saito H, Nakagawa F, Tsujimoto H, Sakamoto K, Tsukioka S, Uchida J, Kiniwa M, Kobunai T, Takechi T (2012) Combination therapy using oral S-1 and targeted agents against human tumor xenografts in nude mice. Exp Ther Med 3:755–762
Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ (2000) Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer 89:74–82
Kobunai T, Waranabe T, Fukusato T (2011) Antitumor activity of S-1 in combination with Cetuximab on human gastric cancer cell lines in vivo. Anticancer Res 31:3691–3696
Fukuda K, Saikawa Y, Takahashi M, Takahashi T, Wada N, Kawakubo H, Takeuchi H, Kitagawa Y (2012) Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. Int J Oncol 40:975–982
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39:2–15
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. J Clin Oncol 25:1658–1664
André T, Blons H, Mabro M, Chibaudel B, Bachet JB, Tournigand C, Bennamoun M, Artru P, Nguyen S, Ebenezer C, Aissat N, Cayre A, Penault-Llorca F, Laurent-Puig P, de Gramont A, GERCOR (2013) Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol 24:412–419
Shitara K, Yokota T, Takahari D, Shibata T, Ura T, Utsunomiya S, Inaba Y, Yamaura H, Sato Y, Najima M, Kawai H, Tajika M, Sawaki A, Yatabe Y, Muro K (2011) Phase II study of combination chemotherapy with irinotecan and cetuximab for pretreated metastatic colorectal cancer harboring wild-type KRAS. Invest New Drugs 29:688–693
Shitara K, Yuki S, Yoshida M, Takahari D, Utsunomiya S, Yokota T, Sato Y, Inaba Y, Tajika M, Kawai H, Yamaura H, Kato M, Yamazaki K, Komatsu Y, Muro K (2012) Phase II study of combination chemotherapy with biweekly cetuximab and irinotecan for wild-type KRAS metastatic colorectal cancer refractory to irinotecan, oxaliplatin, and fluoropyrimidines. Invest New Drugs 30:787–793
Tahara M, Shirao K, Boku N, Yamaguchi K, Komatsu Y, Inaba Y, Arai T, Mizunuma N, Satoh T, Takiuchi H, Nishina T, Sakata Y (2008) Multicenter phase II study of cetuximab plus irinotecan in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrimidines. Jpn J Clin Oncol 38:762–769
Muro K, Yoshino T, Doi T, Shirao K, Takiuchi H, Hamamoto Y, Watanabe H, Yang BB, Asahi D (2009) A Phase 2 clinical trial of panitumumab monotherapy in Japanese patient as with metastatic Colorectal Cancer. Jpn J Clin Oncol 39:321–326
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA 3rd (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319
Oki E, Emi Y, Miyamoto Y, Kabashima A, Higashi H, Ogata Y, Ikebe M, Saeki H, Tokunaga S, Shirabe K, Beppu T, Uchida S, Takatsuki M, Sakoda M, Eguchi S, Akagi Y, Kakeji Y, Baba H, Natsugoe S, Maehara Y, Kyushu Study Group of Clinical Cancer (KSCC) (2015) Phase II trial of S-1 and oxaliplatin plus cetuximab for colorectal cancer patients with initially unresectable or not optimally resectable liver metastases (KSCC1002). Ann Surg Oncol 22:S1067–S1074
Ogawa M, Anan T, Suzuki T, Okuma M, Ichihara K, Hasegawa T, Yoshida K, Yanaga K (2016) Initial report of phase II study on bi-weekly SOX plus cetuximab treatment for wild-type K-RAS advanced and recurrent colorectal cancer. Anticancer Res 36:2505–2511
Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T, Saito K, Zergebel C, Rosen LS (2011) Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 102:478–483
Acknowledgments
The KSCC0901 was conducted by the KSCC and Clinical Research Support Center (CReS) Kyushu. Merck Serono and Taiho Pharmaceutical Co. provided an unrestricted contribution to CReS Kyushu. We greatly thank the participated patients and their families. We are indebted to the physicians and all of the clinical study teams at the participating institutions. We thank all other co-medical staff, and the Independent Data Monitoring Committee (Kuniaki Shirao, Toshiro Kuroiwa, Yoichi Nakanishi, Shuji Nakano, and Eishi Baba) who contributed to this study. We also thank Ms. Taniguchi, Ms. Sakamoto, Ms. Shimamoto, Mr. Aratani, and the other staff at CReS Kyushu for their excellent collection and manage of data, secretarial assistance, and other support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
H. Baba and K. Yoshida have received research grants from Merck Serono and Taiho Pharmaceutical Co., Ltd. T. Takahashi, E. Oki, A. Tsuji, H. Baba, K. Yoshida and Yoshihiko Maehara have received speaker honorarium from Merck Serono and Taiho Pharmaceutical Co., Ltd.. Y. Emi, K. Kobayashi, M. Shimokawa, T. Tanaka, Y. Akagi, Y. Ogata, S. Natsugoe declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takahashi, T., Emi, Y., Oki, E. et al. Multicenter phase II study of combination therapy with cetuximab and S-1 in patients with KRAS exon 2 wild-type unresectable colorectal cancer previously treated with irinotecan, oxaliplatin, and fluoropyrimidines (KSCC 0901 study). Cancer Chemother Pharmacol 78, 585–593 (2016). https://doi.org/10.1007/s00280-016-3109-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3109-4