Abstract
Purpose
Pasireotide LAR (SOM230 LAR) is a cyclohexapeptide engineered to bind to multiple somatostatin receptor subtypes to mimic the action of naturally occurring somatostatin with higher affinity to these receptors than octreotide and is a potent inhibitor of insulin-like growth factor-1 (IGF-1). Somatostatin receptors and IGF receptors are highly expressed in pancreatic cancer, thereby potentially making it a valuable target. This phase I study evaluated safety, tolerability and preliminary tumor response of pasireotide LAR in combination with gemcitabine in locally advanced or metastatic pancreatic cancer.
Methods
Patients with previously untreated metastatic pancreatic cancer were included. A 3 + 3 dose-escalation design was used. Patients received gemcitabine on days 1, 8 and 15 and pasireotide LAR IM monthly in a 28-day cycle. Two dose levels of pasireotide LAR were planned: 40 mg IM and 60 mg. Cohort was expanded by ten more patients at the highest tested dose to further assess the safety and efficacy.
Results
Twenty patients were consented on this trial, and 16 patients were evaluable for safety and efficacy. No dose-limiting toxicities were observed. Two out sixteen patients (12 %) had partial response, and nine of sixteen (56 %) had stable disease as best response. Median progression-free survival was 4.1 months (range 1–16 months), and median overall survival was 6.9 months (range 1–25 months). Most common grade 3 or 4 toxicities were hyperglycemia (n = 5), hyperbilirubinemia (n = 1) and thrombocytopenia (n = 2). Median baseline IGF-1 level was lower in patients with stable disease than in those with progressive disease (63 vs 71 ng/ml).
Conclusion
Pasireotide in combination with gemcitabine was well tolerated with disease control rate of 68 %. Larger trials are needed in the future to establish its efficacy in the treatment of pancreatic cancer.
Clinical trial
NCT01385956.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a lethal disease and represents the fourth leading cause of cancer-related death in the USA [1]. Majority of patients with pancreatic adenocarcinoma are initially diagnosed as locally advanced or metastatic stage at diagnosis, thereby leading to dismal prognosis and 5-year survival rate of 6 % [2]. Gemcitabine has been the only approved single-agent therapy for a long time, with a median survival of 5.7 months [3]. Recently, the new combination of gemcitabine plus nab-paclitaxel and FOLFIRINOX (5-fluorouracil, irinotecan and oxaliplatin) has shown improvement in overall survival compared to gemcitabine alone in the treatment of metastatic pancreatic cancer [2, 4]. Gemcitabine plus nab-paclitaxel improved overall survival from 6.7 to 8.5 months (P = 0.000015), and FOLFIRINOX improved overall survival from 6.8 to 11.1 months (P < 0.001) compared to gemcitabine alone. However, the combination regimens were associated with higher grade 3 and 4 toxicities such as myelosuppression, diarrhea and sensory neuropathy. Therefore, there is still a need to develop novel approaches for treatment of pancreatic cancer.
Pasireotide LAR is the next generation in somatostatin analogs with a high binding affinity for four of the five known somatostatin receptor subtypes (sst1, sst2, sst3 and sst5) which are expressed in a variety of tumors including breast, prostate, ovarian, colon and pancreatic cancer. Compared with endogenous somatostatin, pasireotide has a twofold higher affinity for sst5 and compared with octreotide; it has a 40-, 30- and fivefold higher binding affinity for sst5, sst1 and sst3, respectively, and a slightly lower (0.4 times lower) binding affinity for sst2 [5, 6]. Preclinical studies demonstrate that somatostatin analogs have antiproliferative activity on a variety of tumors, through a various direct and indirect mechanisms. Direct antitumor activities are mediated through somatostatin receptors and include antimitotic and apoptotic effects. The antimitotic activity was shown to happen via blocking cell cycle progression by arresting cells at the G1/S phase primarily through sst1, sst2, sst4 and sst5 or the G2/M phase through sst3 [7, 8], whereas inducing apoptosis attributed mainly to sst2 and sst3 [9]. Indirect antitumor activities of somatostatin analogs are mediated via inhibiting angiogenesis and IGF-1 production. Angiogenesis suppression occurs primarily through sst1 and sst3, which are highly expressed in blood vessels. Sst1 has inhibitory effect on endothelial proliferation and neovascularization [10], whereas SSRT3 inhibits the transcription of vascular endothelial growth factor (VEGF) [11]. Pasireotide decreases IGF-1 production by inhibiting both GH synthesis in pituitary gland and GH action in the liver, leading to IGF-1 transcription downregulation in the liver [12, 13]. Insulin-like growth factor-I receptor (IGF-IR) is frequently over-expressed and constitutively activated in pancreatic cancer, and it possesses tyrosine kinase activity [14]. The disruption of IGF-1 production and signaling via somatostatin analogs leads to the inhibition of multiple key downstream signaling pathways such as AKT/PI3 K, MAPK, JAK/STAT and EMT which ultimately reduce tumor growth and motility of cancer cells [15, 16].
Apparent synergistic effect of IGF-I inhibitors with gemcitabine in vitro on multiple cancer lines was recognized by affecting S phase and G1 phase [17]. Thus, pasireotide with its high binding affinity to somatostatin receptor subtypes, its potent inhibitory effects on IGF-I release and the potential synergistic antitumor effects with gemcitabine represent a valuable targeted therapy and a promising treatment for patients with pancreatic cancer. We conducted a phase I study to assess the safety and efficacy of pasireotide and gemcitabine in patients with locally advanced or metastatic pancreatic cancer.
Materials and methods
Patient population
All patients were required to be 18 years of age or older and have histologically confirmed evidence of epithelial cancer (adenocarcinoma) of the exocrine pancreas. Only patients with metastatic or locally advanced pancreatic cancer with ECOG performance status ≥2 were included. Treatment with prior chemotherapy was allowed if it was with gemcitabine alone or 5-FU with radiation as an adjuvant therapy and occurred more than 6 months. Mandated laboratory requirements included: serum bilirubin ≤2× upper limit of normal (ULN) and serum transaminases activity ≤3× ULN, with the exception of serum transaminases (<5× ULN) if the patient has liver metastases, serum creatinine ≤1.5× ULN, absolute neutrophil count ≥1.0 × 109/l, platelets ≥100 × 109/l, Hgb > 9 g/dl, fasting serum cholesterol ≤300 mg/dl or ≤7.75 mmol/l and fasting triglycerides ≤2.5× ULN with appropriate lipid-lowering medications. Patients with history of clinically significant cardiac arrhythmias, prolonged corrected QT (QTc) interval at screening (>450 ms) or on medications known to prolong QTc were excluded. All patients provided written informed consent for participation in the study, which was approved by the institutional review boards at H. Lee Moffitt Cancer Center.
Study design
This was a single-arm, open-label, phase I study of combination therapy with pasireotide LAR and standard treatment with gemcitabine 1000 mg/m2 weekly ×3 and then 1 week off. Pasireotide LAR at doses of 20, 40 and 60 mg has been found to be generally well tolerated in carcinoid and acromegalic patients [18]. Since this was the first trial using pasireotide LAR in combination with gemcitabine, we utilized a staggered, sequential (3 + 3) dose-escalation design to define the MTD of pasireotide LAR when combining with gemcitabine. Two dose levels (DL) were planned: 40 and 60 mg IM, once every 28 days, by deep intragluteal injection. The MTD of pasireotide LAR was defined as the highest dose level at which no more than one out of six subjects experiences dose-limiting toxicity (DLT). DLT was defined as grade 3 or higher non-hematological toxicity (excluding alopecia), febrile neutropenia (absolute neutrophil count <1000/µl and fever >101 F), grade 4 neutropenia lasting for more than 7 days or grade 3 or 4 fasting hyperglycemia persisting for at least 5 days in spite of optimal medical management. The cohort was expanded by ten more patients to assess safety and efficacy following MTD determination.
End points and response assessments
The primary goal of this trial was to determine the maximum tolerated dose (MTD) of pasireotide LAR in combination with standard doses of gemcitabine. Secondary goals were to assess objective tumor response, progression-free survival and overall survival. As an exploratory analysis, we also assessed the association between best response and levels of baseline circulating IGF-I, insulin-like growth factor-binding protein 1 (IGFBP-1) and IGFBP-3.
Tumor response assessment utilizing CT of chest, abdomen and pelvis or MRI was completed on all assessments at screening and every 2 cycles thereafter until tumor progression. Standard response evaluation criteria in solid tumors (RECIST) guidelines version 1.1 was used to assess response.
Biomarker analyses
All serum biomarkers were collected from blood 30–60 min prior to injection of pasireotide LAR and were checked every 8 weeks until progression. The biomarker concentrations were measured by enzyme-linked immunosorbent assay (ELISA) with reagents from Diagnostic Systems Laboratories (DSL, Webster, TX, USA) [19]. IGF-I assay utilized an acid–ethanol precipitation of IGF-I-binding proteins, to avoid interference of IGFBPs with the IGF-I assay. Laboratory personnel were unable to distinguish between case and control samples. Three different quality-controlled serum samples were used in each batch, and 5 % blinded duplicate samples were included for quality control. The mean intrabatch coefficients of variation from the quality-controlled samples were estimated to be 3.0 % (at 5.72 nmol/l) for IGF-I and 5.3 % (at 141 nmol/l) for IGFBP-3. Interbatch coefficients of variations were 13.7 % for IGF-I concentration of 13 nmol/l and 9.4 % for IGFBP-3 concentration of 140 nmol/l.
Safety
Fasting serum chemistries and hematological parameters for toxicity were checked with each dose of chemotherapy. Patients were evaluated with a physical examination before each cycle of treatment. ECGs to measure QTc were obtained prior to starting the study and on day 22 of each cycle. Glycosylated hemoglobin was measured on day 22 of each cycle starting from second cycle.
Statistical analysis
Descriptive statistics were used to characterize demographic and clinical factors. Kaplan–Meier curves were created for both progression-free survival and overall survival. Progression-free survival was defined as the duration of time from start of treatment to time of progression or death, whichever comes first. Overall survival will be calculated from date of registration to the date of death or date of last follow-up. For the patients to be considered evaluable for efficacy, they must have undergone at least one restaging scan. For toxicity assessment, all patients who received at least one dose were included. Biomarkers concentration was correlated with the clinical responses of the patients in the expanded cohort. All statistical analyses were performed using SAS (version 9.3; SAS Institute; Cary, NC, USA).
Results
Patient characteristics
Twenty patients consented for the study, but four patients withdrew consent before starting treatment primarily due to geographical relocation. Sixteen patients were evaluated for safety and efficacy. All patients had metastatic pancreatic cancer at presentation with ECOG performance status of one. None of the patient had received prior chemotherapy or surgery for pancreatic malignancy. Median age was 65.5 years (range 55–85 years): six males and ten females. Three patients were enrolled in each of the two cohorts: DL1 (40 mg of pasireotide LAR) and DL2 (60 mg of pasireotide LAR). Pasireotide LAR at 60 mg monthly IM injection with weekly gemcitabine was expanded to ten patients to assess safety and efficacy (Table 1).
Safety and toxicities
Suspected treatment-related adverse events were consistent with the expected toxicities of both gemcitabine and pasireotide (Table 2). Grade 3 or 4 hyperglycemia was seen in five of the 16 evaluated patients. Hyperglycemia was managed with oral hypoglycemic agents, and three patients required insulin injections. Grade 3 or 4 hematological toxicities included thrombocytopenia (two patients) and neutropenia (one patient). Other grade 3 or 4 non-hematological toxicities included two patients with fatigue and one patient with increase bilirubin. QTc prolongation was not observed in any patients in this study.
Efficacy
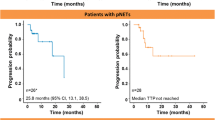
Patients were followed up for radiological responses with cross-sectional imaging studies after every 8 weeks of treatment. We observed partial responses (PR) in two of the sixteen patients (12 %), and nine patients (56 %) experienced stable disease (SD) as their best response to therapy by RECIST. Five patients (31 %) had progressive disease (PD) at the first restaging scans. Best overall response is illustrated by the waterfall plot in Fig. 1. Median PFS for all patients was 4.1 months (range 1–16 months) (Fig. 2), and median overall survival was 6.9 months (range 1–25 months) (Fig. 3). Estimated 6-month PFS rate was 31 % (95 % CI 0.11, 0.54), and 6-month OS rate was 63 % (95 % CI 0.35–0.81). Four patients had an overall survival longer than 14 months (14, 16, 21 and 25 months). The last patient is still alive receiving different chemotherapy regimen by the time this manuscript is being written. The median baseline levels of IGF-1, IGFBP-1 and IGFBP-3 were 69.20 ng/ml (range 55.40–142.70), 5.15 ng/ml (range 0.85–54.74) and 2246 ng/ml (range 1799–3219), respectively (Table 3). Interestingly, the median baseline levels of IGF-1, IGFBP-1 and IGFBP-3 were lower in patients with stable disease than in those with progressive disease (63.25 vs 71.70 ng/ml), (5.15 vs 8.36 ng/ml), and (2051.50 vs 2568.00 ng/ml), respectively. However, this did not reach statistical significance likely due to small numbers.
The median decrease in the levels of IGF-1 and IGFBP-3 between the first cycle and the last cycle was 22.63 and 1080.63 ng/ml, respectively. The change in levels of IGF-1, IGFBP-1 and IGFBP-3 during the study was not statically significant with response to treatment likely due to small study size too, P = 0.11, 0.32 and 0.07, respectively.
Discussion
This is the first study to evaluate the safety and tolerability of pasireotide LAR combined with gemcitabine in previously untreated metastatic pancreatic cancer. Our phase I study demonstrated the feasibility of combining the novel long-acting somatostatin analog pasireotide with gemcitabine in patients with metastatic pancreatic cancer. Pasireotide and gemcitabine regimens were relatively well tolerated, and no deaths related to the study were reported. The adverse events associated with this regimen were consistent with the anticipated toxicities of either agent alone. No patients discontinued the study treatment due to adverse events. No dose-limiting toxicities related to pasireotide were noted at any dose level. Only few grade 3–4 toxicities were observed in this study, and these toxicities were fatigue, neutropenia, thrombocytopenia and hyperglycemia (Table 2). All these side effects except the hyperglycemia were attributed to gemcitabine. The hyperglycemia was noted in five patients (30 %) in our study, and it is one of the well-known and major toxicities of pasireotide. Hyperglycemia is attributed to the indirect suppression of glucagon-like peptide (GLP-1) by pasireotide, resulting in stimulation of glucagon secretion [20]. Cardiac bradycardia and QTc prolongation have been reported previously as adverse events of pasireotide [21]. QTc interval was monitored closely in our study, and no prolongation of QTc was observed with pasireotide LAR at dose of 40 or 60 mg IM monthly. Although assessment of efficacy was not a primary objective of our study, the combination of pasireotide and gemcitabine showed potential activity with disease control rate of 68 % (56 % SD + 12 % PR).
IGF pathway seems to play critical role in carcinogenesis of pancreatic cancer. Tian et al. described the inhibitory effect of blocking IGF-IR on pancreatic cancer cells colony formation and xenograft tumor growth genesis by suppressing PI3 K/AKT and NF-kB pathways and increasing mitochondrial-mediated apoptosis [22]. Hirakawa et al. [23] noted that IGF-I receptors expression was associated with significantly poorer survival and poorly differentiated tumors in 122 patients with pancreatic cancer. In our study, the baseline of IGF-1 was lower in patients with stable disease than in patients with progressive disease, and the median drop of IGF-1 level between the first cycle and the last cycle was more prominent in the stable disease than in progressive disease which affirms the importance of this pathway in pancreatic cancer. Insulin-like growth factor-binding proteins (IGFBP-1 to IGFBP-6) serve as a carrier protein for insulin-like growth factor-1 (IGF-1) in the serum and regulate its activity by reducing the bioavailability of IGF-1 to bind to its cellular receptors. Independent of IGF-1-binding capacity, IGFBPs also function in the pericellular and intracellular compartments and regulate cell growth and survival by their involvement in transcriptional regulation, induction of apoptosis and DNA damage repair [24]. In our study, the baseline of IGFBP-1 and IGFBP-3 levels was lower in patients with stable disease than in patients with progressive disease. Thus, these biomarkers may represent potential prognostic factors, but larger studies are needed in the future to confirm and validate these findings.
Ganitumab which is an investigational, fully human, mAb IGF-IR inhibitor had failed to demonstrate a statistically significant improvement in overall survival when it was combined with gemcitabine in phase III GAMMA clinical trial comparing to placebo as first-line therapy for metastatic adenocarcinoma of the pancreas [25]. However, pasireotide might represent a potential promising treatment for pancreatic cancer because in addition to its role in disrupting IGF pathway by inhibiting IGF production, pasireotide directly affects the somatostatin receptors (sst). By targeting those receptors, the drug can control cancer cell proliferation through the interference with different signaling pathways (PTPs, JAK2, Ras/ERK and Pi3 K/Akt), resulting in cytostatic effects mediated by the induction of cell cycle inhibitors p27 or p21, or tumor suppressors, such as Zac1 [26]. However, the role of sst inhibition in carcinoma remains unclear. In this study, we did not check sst, but checking the sst in future trials may be warranted to better understand predictive or prognostic value of the receptors.
Although we did not check pharmacokinetics in this study, we believe that our study demonstrated that the combination of gemcitabine and pasireotide was very well tolerated. Of note, dose of pasireotide was stopped before the MTD was reached; thus, higher dose of pasireotide may have greater efficacy. Consideration should be given to investigate this potent multi-receptor-targeting somatostatin analog with modern chemotherapy regimens including FOLFIRINOX and gemcitabine/nab-paclitaxel which represent the standard of care in advanced pancreatic cancer.
References
Jemal A et al (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Conroy T et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Burris HA III et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Von Hoff DD et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703
Bruns C et al (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146(5):707–716
Beglinger C et al (2012) Multiple once-daily subcutaneous doses of pasireotide were well tolerated in healthy male volunteers: a randomized, double-blind, placebo-controlled, cross-over, phase I study. Endocrine 42(2):366–374
Cheung NW, Boyages SC (1995) Somatostatin-14 and its analog octreotide exert a cytostatic effect on GH3 rat pituitary tumor cell proliferation via a transient G0/G1 cell cycle block. Endocrinology 136(10):4174–4181
Srikant CB (1995) Cell cycle dependent induction of apoptosis by somatostatin analog SMS 201-995 in AtT-20 mouse pituitary cells. Biochem Biophys Res Commun 209(2):400–406
Ferrante E et al (2006) Octreotide promotes apoptosis in human somatotroph tumor cells by activating somatostatin receptor type 2. Endocr Relat Cancer 13(3):955–962
Albini A et al (1999) Somatostatin controls Kaposi’s sarcoma tumor growth through inhibition of angiogenesis. FASEB J 13(6):647–655
Bocci G et al (2007) In vitro antiangiogenic activity of selective somatostatin subtype-1 receptor agonists. Eur J Clin Invest 37(9):700–708
Murray RD et al (2004) Central and peripheral actions of somatostatin on the growth hormone-IGF-I axis. J Clin Invest 114(3):349–356
Theodoropoulou M, Stalla GK (2013) Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 34(3):228–252
Bergmann U et al (1995) Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res 55(10):2007–2011
Okusaka T et al (2014) Safety, tolerability, pharmacokinetics and antitumor activity of ganitumab, an investigational fully human monoclonal antibody to insulin-like growth factor type 1 receptor, combined with gemcitabine as first-line therapy in patients with metastatic pancreatic cancer: a phase 1b study. Jpn J Clin Oncol 44(5):442–447
Sara VR, Hall K (1990) Insulin-like growth factors and their binding proteins. Physiol Rev 70(3):591–614
Wolf S et al (2010) Treatment of biliary tract cancer with NVP-AEW541: mechanisms of action and resistance. World J Gastroenterol 16(2):156–166
Wolin EM, Hu K, Hughes G, Bouillaud E, Giannone V, Resendiz KH (2013) Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother Pharmacol 72(2):387–395
Kaaks R et al (2000) Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92(19):1592–1600
Holst JJ et al (2011) Regulation of glucagon secretion by incretins. Diabetes Obes Metab 13(Suppl 1):89–94
Herrington AM, George KW, Moulds CC (1998) Octreotide-induced bradycardia. Pharmacotherapy 18(2):413–416
Tian X et al (2013) Insulin-like growth factor 1 receptor promotes the growth and chemoresistance of pancreatic cancer. Dig Dis Sci 58(9):2705–2712
Hirakawa T et al (2013) IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC Cancer 13:392
Baxter RC (2014) IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 14(5):329–341
Fuchs CS et al (2015) A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trialdagger. Ann Oncol 26(5):921–927
Barbieri F et al (2013) Peptide receptor targeting in cancer: the somatostatin paradigm. Int J Pept 2013:926295
Acknowledgments
This work was supported in part by a grant from Novartis, which provided funding as well as study drug for participants. The manuscript was reviewed and approved prior to publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Suleiman, Y., Mahipal, A., Shibata, D. et al. Phase I study of combination of pasireotide LAR + gemcitabine in locally advanced or metastatic pancreatic cancer. Cancer Chemother Pharmacol 76, 481–487 (2015). https://doi.org/10.1007/s00280-015-2814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2814-8