Abstract
Purpose
Although epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR–TKIs) have become key therapeutic agents for non-small cell lung cancer (NSCLC) patients with EGFR mutation, little is known about the efficacy of EGFR–TKIs according to different treatment timings.
Methods
A total of 1,250 patients with NSCLC were screened for EGFR mutations at a single institution between March 2006 and May 2010. The efficacy of EGFR–TKIs in terms of response rate (RR), progression-free survival (PFS), and overall survival (OS) were compared according to the treatment timing.
Results
Among the 437 patients (36.1 %) with EGFR mutation, we analyzed 222 patients who received EGFR–TKI treatment. With a median follow-up duration of 27.5 months (range 8.3–69.2), EGFR–TKI was given to 97 (43.7 %), 109 (49.1 %), and 16 (7.2 %) patients as first-line, second-line, and third-line therapy, respectively. All three groups showed similar RR (71.1, 72.5, and 75.0 %, respectively) to EGFR–TKI (p = 0.802). No significant difference was observed according to treatment timing of EGFR–TKI in terms of PFS (median 10.6, 13.0, and 10.4 months; p = 0.670) and OS (median 20.5, 26.2, and 17.1 months; p = 0.142). The treatment timing of EGFR–TKI still showed no association with PFS or OS after adjusting significant prognostic factors including performance, disease status, and EGFR mutation types.
Conclusions
EGFR–TKIs showed similar efficacy in patients with EGFR mutation-positive adenocarcinoma in terms of RR, PFS, and OS irrespective of treatment timing. Although EGFR–TKIs are currently the treatment of choice of first-line treatment in patients with EGFR-positive tumors, the sequential treatment with EGFR–TKI could be a reasonable option when EGFR mutation status cannot be obtained in a short time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several targeted therapies against epidermal growth factor receptor (EGFR) have been developed and EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib have been shown to be effective in patients with advanced non-small cell lung cancer (NSCLC) [1, 2]. Because EGFR-activating mutations are a predictor of the efficacy of EGFR–TKI treatment, an EGFR mutation-based approach has contributed to the advance of personalized therapy [3, 4]. However, the success of personalized therapy is dependent on not only the target of the treatment, but also the sequence of treatments and little is known about the efficacy of EGFR–TKIs according to treatment timing.
As a result of the notable improvement in survival outcome achieved in patients with EGFR-positive tumors in several phase III clinical trials [1, 5–7], current guidelines recommend EGFR–TKI therapy as the first-line treatment based on a higher response rate (RR), longer progression-free survival (PFS), and better quality of life (QOL) compared with first-line chemotherapy, although first-line EGFR–TKIs did not improve overall survival (OS) [8, 9]. The Spanish Lung Cancer Group (SLCG) reported similar responses to EGFR–TKIs in patients with EGFR mutation-positive tumors regardless of whether they were administered as first-line or second-line therapy [10]. However, the improvement in PFS with first-line EGFR–TKI observed in all previous phase III studies did not result in improved OS. In addition, direct sequencing, the classic method for EGFR testing, usually takes about 2 weeks after pathologic confirmation [4].
In practice, EGFR–TKI is frequently used as second-line or more sequential treatment when the results of EGFR mutation testing cannot be obtained in a short period of time. Therefore, we explored the current timing of treatment with EGFR–TKI and evaluated efficacy according to the timing of EGFR–TKI in a clinical practice. We also identified prognostic factors in patients with lung adenocarcinoma that possess EGFR mutations.
Materials and methods
Patients
We reviewed 1,250 patients who were screened by EGFR mutation sequencing between March 2006 and May 2010 at Asan Medical Center (Seoul, Korea). All patients were ≥18 years of age and had histologically confirmed NSCLC. After exclusion of 39 patients with specimens that were inadequate for sequencing, 473 patients (36.1 %) were shown to have EGFR mutations. Further, 150 patients were excluded for the following reasons: non-adenocarcinoma (to minimize the influence of histological type; n = 17), history of other malignancy (n = 27), no treatment (n = 30), and no evidence of disease after curative resection (n = 76). Among the 287 patients who had advanced-stage (metastatic or recurrent) lung adenocarcinoma with proven EGFR mutation, 222 patients who were treated with EGFR–TKI were retrospectively evaluated (Fig. 1). At the time of analysis, 65 patients had not received EGFR–TKI because they were receiving cytotoxic chemotherapy as first-line treatment (n = 46) or EGFR–TKI was deferred with close follow-up after radiotherapy or palliative surgery (n = 19). The timing and order of the various chemotherapy regimens depended on the physicians’ decision after discussion with the patient. The patients’ medical records were reviewed for information regarding demographic data, tumor characteristics, treatment types, treatment responses, and survival. The study was approved by the Institutional Review Board of the Asan Medical Center.
Molecular analysis of EGFR mutation
Tumor specimens, including paraffin blocks or frozen tissues of surgical specimens, and core needle biopsies were used for mutational analysis. Macro-dissection of the tumor area from the slide was carried out to obtain sufficient material and to decrease the risk of false negatives [11]. DNA was isolated from tumors embedded in paraffin blocks using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). Exons 18–21 of the EGFR coding sequence encoding the tyrosine kinase domain were amplified by polymerase chain reaction (PCR) using primers and PCR conditions as reported [12]. Independent amplifications were purified and sequenced in an automatic ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA, USA). All sequencing reactions were performed in forward and reverse directions, using tracings from at least two amplifications.
Treatment and evaluation of response
Patients were treated with the EGFR–TKIs gefitinib or erlotinib according to the physicians’ preference. Gefitinib was taken orally at a dosage of 250 mg daily and erlotinib was taken orally at a dosage of 150 mg daily. Baseline assessments were usually performed within 2 weeks before treatment. A chest computed tomography scan (including liver and adrenal glands) was performed every 2 or 3 months as routine clinical practice and as needed to confirm the response and progression of disease. Tumor response was classified according to the response evaluation criteria in Solid Tumors 1.0 guidelines [13]. Confirmation of the response was not required for this study because it was a retrospective analysis.
Statistical analyses
OS and PFS were calculated from the date of EGFR–TKI administration until death from any cause or censoring at last follow-up and the first sign of disease progression, respectively. In addition, total OS (OST) was calculated from the date of first-line antitumor therapy, either EGFR–TKI or another chemotherapy regimen, until death from any cause or censoring at last follow-up. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard regression model. Differences in baseline clinical characteristics between patients groups according to timing of EGFR–TKI were compared using the t test or Mann–Whitney test for continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables, as appropriate. A two-sided p value <0.05 was considered significant, and 95 % confidence intervals (CIs) were calculated. All statistical analyses were performed using the SPSS 18.0 software package (IBM SPSS Statistics, USA).
Results
Baseline characteristics
Of the 222 patients with EGFR mutation-positive lung adenocarcinoma, 67 (30.2 %) were male. The median age at EGFR–TKI treatment was 59 years (range 33–86 years), and 165 patients (74.3 %) were never smokers. The EGFR mutations identified were classified as deletion in exon 19 (del 19; 117 patients, 52.7 %), missense mutation in exon 21 (L858R; 86 patients, 38.7 %), or other mutations (either single or complex mutation; 19 patients, 8.6 %).
Comparison of the baseline characteristics of patients according to the timing of EGFR–TKI as first-line, second-line, or third-line treatment is summarized in Table 1. All patients who were treated with EGFR–TKI as second- or third-line treatment started to receive EGFR–TKI after disease progression that occurred during or after prior conventional chemotherapy. Compared with patients who received EGFR–TKI as second-line (second-line group) or third-line therapy (third-line group), the 97 patients who received EGFR–TKI as first-line therapy (first-line group) tended to be older, female, never smokers, and had poorer Eastern Cooperative Oncology Group (ECOG) performance. In addition, the ratio of patients exposed to platinum-doublet chemotherapy was significantly lower in first-line group compared with second-line group or third-line group (p < 0.001). There was no significant difference in histological type and mutation type among the three groups.
Response according to the timing of EGFR–TKI
Eleven patients (5.0 %) achieved a complete response and 149 patients (67.1 %) showed partial response irrespective of the timing of EGFR–TKI, yielding an overall RR of 72.1 % (95 % CI, 66.2–78.0 %; Table 2). Forty-five patients (20.2 %) had stable disease, and seven (3.2 %) had progressive disease. Ten patients (4.5 %) were not evaluable for tumor response because of loss to follow-up after the first treatment cycle of EGFR–TKI. There was no statistical significant difference in terms of RR among the three groups (p = 0.802): first-line (71.1 %; 95 % CI, 62.1–80.1), second-line (72.5 %; 95 % CI, 64.1–80.9), and third-line (75.0 %; 95 % CI, 53.8–96.2).
Survival outcome according to the timing of EGFR–TKI
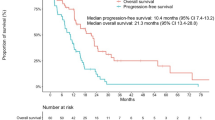
At the time of analysis, the median follow-up duration was 22.3 months (range 5.1–63.6 months). The median PFS and OS after EGFR–TKI were 12.5 months (95 % CI, 10.5–14.4 months) and 22.6 months (95 % CI, 18.2–27.0 months), respectively. PFS and OS did not differ significantly among the three groups. Median PFS of first-line, second-line, and third-line groups was 10.6 months (95 % CI, 7.2–14.0), 13.0 months (95 % CI, 11.5–14.6), and 10.4 months (95 % CI, 4.3–16.5), respectively (p = 0.670, Fig. 2). Median OS of each group was 20.5 months (95 % CI, 15.0–25.9), 26.2 months (19.7–32.8), and 17.1 months (95 % CI, 6.1–28.1), respectively (p = 0.142). Median OST (total survival time from the first-line antitumor therapy) of all patients was 26.7 months (95 % CI, 21.7–31.6 months), and there was no significant difference in OST among the three groups (p = 0.132). When the OST of first-line group (20.5 months, 95 % CI, 15.0–25.9) was compared with the OST of second-line (32.1 months, 95 % CI, 23.3–41.0) or third-line group (27.9 months, 95 % CI, 16.9–38.9), there was a borderline significant difference between first-line and second-line groups (p = 0.050), but no significant difference between first-line and third-line groups (p = 0.726).
Univariate and multivariate analysis
Univariate analysis of factors affecting patient survival is shown in Table 2. Performance status (ECOG PS 0–1 vs. 2–3), disease status (recurrent vs. metastatic), histology (bronchioloalveolar carcinoma vs. adenocarcinoma), and type of EGFR mutation (del 19 or L858R vs. others) were significant prognostic factors for PFS and OS. Multivariate analysis showed that poor ECOG PS, metastatic disease, and uncommon EGFR mutation (other than del 19 or L858R) were independent prognostic factors for poor PFS and OS (Table 3). The treatment timing of EGFR–TKI still showed no association with PFS or OS after adjusting significant prognostic factors.
Discussion
In this study population, the EGFR mutation rate was 36.1 % and mutations in exon 19 and 21 accounted for 91.4 % of all EGFR mutations. The overall RR to EGFR–TKIs was 72.1 % and the median PFS was 12.5 months, similar to previous Asian studies [14, 15]. EGFR–TKIs showed similar efficacy regardless of treatment timing in terms of RR (71–75 %), PFS (10.4–13.0 months), and OS (17.1–26.2 months). This study suggested that the third-line EGFR–TKI also had similar efficacy to the first or second-line EGFR–TKI, although the number of patients who received EGFR–TKI is small.
Recent developments in NSCLC therapy have allowed physicians to treat patients with a personalized approach according to histology, EGFR mutation status, or the presence of biomarkers [16]. For first-line chemotherapy, there is no significant difference in survival among four commonly used regimens including cisplatin/paclitaxel, cisplatin/gemcitabine, cisplatin/docetaxel, or carboplatin/paclitaxel [17]. The Iressa Pan-Asia Study (IPASS), a representative study comparing EGFR–TKI therapy with chemotherapy, was designed on the basis of molecular characteristics [7]. In EGFR mutation-positive tumors, gefitinib showed superiority for PFS compared with chemotherapy including carboplatin–paclitaxel [7]. However, the final survival data of IPASS study showed no OS benefit for gefitinib in the EGFR mutation-positive population (HR 1.00; 95 % CI, 0.76–1.33; p = 0.990) [18]. Similarly, other phase III studies involving patients with EGFR-positive NSCLC did not result in improved OS despite an improvement in PFS with first-line EGFR–TKI [1, 5, 6]. This might reflect crossover to sequential EGFR–TKI in all of the trials upon progression of the patients assigned to receive cytotoxic chemotherapy. There has been controversy over which treatment sequence or combination regimen including EGFR–TKI and chemotherapy will have maximal efficacy in terms of OS.
Guidelines recommend that EGFR mutation testing should be performed routinely and EGFR–TKI should be considered for first-line therapy if the patient is found to be EGFR mutation-positive prior to first-line chemotherapy [8, 19]. However, the EGFR mutation testing by the classic method of direct sequencing has the limitation of being time consuming. In practice, direct sequencing after pathologic confirmation usually takes about 2 weeks [4]. Although recent studies of mutant EGFR protein expression by immunohistochemistry are under investigation to overcome the delay in obtaining EGFR status, at the present time, direct sequencing still remains the standard method [20].
Before the EGFR–TKI era, some reports showed that approximately 40–50 % of patients with advanced NSCLC could not receive the sequential treatment after progression on first-line therapy [21]. In our analysis, among the 73 patients who received first-line EGFR–TKI and showed progression, 50 patients (68.5 %) could receive sequential second-line chemotherapy as follows: platinum-based doublet (24 patients, 33 %), single agent (23 patients, 32 %), and others (3 patients). This showed that only 30 % of patients could receive platinum-based doublet chemotherapy after first-line EGFR–TKI. Cytotoxic chemotherapy, especially platinum-based doublet chemotherapy, is still the treatment of choice for NSCLC with no driver mutation and should be administered as soon as possible, especially in patients who are male, smokers, and have non-adenocarcinoma, unless the patients want to wait for the result of EGFR testing. In addition, about 70 % of patients could receive the sequential treatment after progression on first-line therapy in recent study (IPASS) [18].
There might be concern over whether systemic chemotherapy can cause resistance to subsequent EGFR–TKI therapy. Although the RR was not different according to the treatment timing in this study, some studies have shown that the RR to gefitinib in chemotherapy-naive patients was higher than that in patients with prior chemotherapy [22, 23]. However, no general cross-resistance has been demonstrated and EGFR–TKIs are suitable for the treatment of patients with NSCLC who have been treated with platinum-based therapy, particularly those with EGFR mutation-positive tumors [24]. Several studies, including our data, showed no statistical difference between chemotherapy-naive patients and patients who had received prior chemotherapy in terms of PFS or time to progression [10, 22]. In addition, our results showed similar RR and PFS between the patients with first-line EGFR–TKI therapy and patients with one or two courses of prior chemotherapy; in other words, the efficacy of EGFR–TKI was not reduced after a number of prior treatments with cytotoxic agents.
When treating patients with EGFR mutation-positive lung cancer, the physician should consider not only prolonging the OS, but also improving the QOL by personalized therapy. Because the toxicity and safety profile of EGFR–TKI is usually superior to that of cytotoxic chemotherapy, the QOL can be considerably improved [25]. However, the comparison of QOL between EGFR–TKI and chemotherapy should be evaluated for total or sequential treatment periods as well as during first-line treatment. Recently, maintenance chemotherapy with pemetrexed has been established with improved PFS and OS in advanced NSCLC [26]. Also, recent studies showed that only approximately 5 % of patients suffered from grade 3 or higher non-hematologic toxicities in platinum-doublet chemotherapy, and that the toxicity of cytotoxic chemotherapy has been reduced by new treatment options such as maintenance therapy with pemetrexed [27]. Considering the QOL and OS as the sum of PFS, more investigation is needed to maximize the OS benefit at each sequence of treatment in patients with EGFR-mutant NSCLC, including treatment with EGFR–TKIs, maintenance chemotherapy, or newer targeting agents such as next-generation EGFR–TKIs [28], mTOR inhibitor [29], c-met inhibitors [30], or inhibitors of other pathways [31]. Additionally, some studies reported that re-administration of EGFR–TKI was effective and could be a treatment option for patients who once responded to EGFR–TKI and then underwent various subsequent treatments [32, 33].
In this analysis, the patients with uncommon EGFR mutations including exon 18 or 20 had a poor survival outcome (median PFS of 5 months and OS of 8 months). Although the prognostic value of uncommon EGFR mutations remains unclear in NSCLC with EGFR–TKI, several studies also showed that the patients with these uncommon mutations who received EGFR-TKI showed shorter PFS and OS compared with patients with L858R or deletions in exon 19 [34, 35]. Because these uncommon EGFR-mutated NSCLCs are distinctive and heterogeneous, more investigations are needed to develop the novel targeted therapeutic approach in these patients.
This study has some limitations. First, because of the retrospective design, the timing of EGFR–TKI treatment was selected not by protocol, but by the physicians’ decision. Because the EGFR mutation status was unknown at that time, patients who were female, elderly, or poor PS were more likely to be in the first-line group and less exposed to platinum-doublet chemotherapy. Its selection bias might lead a slightly inferior OST in the first-line group compared to the OST of second or third-line group. Second, only patients with adenocarcinoma were included in the analysis to minimize the influence of histological type. The efficacy of EGFR–TKI in other cell types cannot be discussed. Third, our study did not address the QOL of patients during treatment periods. Therefore, we could not compare QOL between EGFR–TKI and cytotoxic chemotherapy according to first-line, second-line, and third-line treatments.
In conclusion, EGFR–TKIs showed similar efficacy in patients with EGFR mutation-positive adenocarcinoma in terms of RR, PFS, and OS regardless of treatment timing. Although EGFR–TKIs are currently the first-line treatment of choice in patients with EGFR-positive NSCLC, the first-line chemotherapy and sequential treatment with EGFR–TKI could be a reasonable option when EGFR mutation status cannot be obtained in a short time. Further prospective studies are needed to evaluate the optimal sequence of treatment regimens including EGFR–TKIs with respect to QOL and improvement of OS.
References
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Novello S, Pimentel FL, Douillard JY, O’Brien M, von Pawel J, Eckardt J, Liepa AM, Simms L, Visseren-Grul C, Paz-Ares L (2010) Safety and resource utilization by non-small cell lung cancer histology: results from the randomized phase III study of pemetrexed plus cisplatin versus gemcitabine plus cisplatin in chemonaive patients with advanced non-small cell lung cancer. J Thorac Oncol 5:1602–1608
Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar J-S, Squire J, Thunnissen E, Ladanyi M (2013) Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 15:415–453
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang J-J, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii56–vii64
Ellis S, Carroll KJ, Pemberton K (2008) Analysis of duration of response in oncology trials. Contemp Clin Trials 29:456–465
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M, The Spanish Lung Cancer Group (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958–967
Eberhard DA, Giaccone G, Johnson BE (2008) Biomarkers of response to epidermal growth factor receptor inhibitors in non-small-cell lung cancer working group: standardization for use in the clinical trial setting. J Clin Oncol 26:983–994
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Inoue A, Suzuki T, Fukuhara T, Maemondo M, Kimura Y, Morikawa N, Watanabe H, Saijo Y, Nukiwa T (2006) Prospective phase II study of gefitinib for chemotherapy-naïve patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24:3340–3346
Sunaga N, Tomizawa Y, Yanagitani N, Iijima H, Kaira K, Shimizu K, Tanaka S, Suga T, Hisada T, Ishizuka T, Saito R, Dobashi K, Mori M (2007) Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer 56:383–389
Pao W, Girard N (2011) New driver mutations in non-small-cell lung cancer. Lancet Oncol 12:175–180
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong S-S, Sriuranpong V, Chao T-Y, Nakagawa K, Chu D-T, Saijo N, Duffield EL, Rukazenkov Y, Speake G, Jiang H, Armour AA, To K-F, Yang JC-H, Mok TSK (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874
National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network (2014) NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version 2.2014 http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 21 Jan 2014
Zhou Q, Zhang X-C, Chen Z-H, Yin X-L, Yang J-J, Xu C-R, Yan H-H, Chen H-J, Su J, Zhong W-Z, Yang X-N, An S-J, Wang B-C, Huang Y-S, Wang Z, Wu Y-L (2011) Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 29:3316–3321
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Wu J-Y, Yu C-J, Yang C-H, Wu S-G, Chiu Y-H, Gow C-H, Chang Y-C, Hsu Y-C, Wei P-F, Shih J-Y, Yang P-C (2008) First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med 178:847–853
Chang GC, Tsai CM, Chen KC, Yu CJ, Shih JY, Yang TY, Lin CP, Hsu JY, Chiu CH, Perng RP, Yang PC, Yang CH (2006) Predictive factors of gefitinib antitumor activity in East Asian advanced non-small cell lung cancer patients. J Thorac Oncol 1:520–525
Murphy M, Stordal B (2011) Erlotinib or gefitinib for the treatment of relapsed platinum pretreated non-small cell lung cancer and ovarian cancer: a systematic review. Drug Resist Updat 14:177–190
Yang JC, Hirsh V, Schuler M, Yamamoto N, O’Byrne KJ, Mok TS, Zazulina V, Shahidi M, Lungershausen J, Massey D, Palmer M, Sequist LV (2013) Symptom control and quality of life in LUX-lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3342–3350
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu Y-L, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. The Lancet 374:1432–1440
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2013) PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31:2895–2902
Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, Ichinose Y, Koboyashi K, Takeda K, Kiura K, Nishio K, Seki Y, Ebisawa R, Shahidi M, Yamamoto N (2013) LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 31:3335–3341
Fei SJ, Zhang XC, Dong S, Cheng H, Zhang YF, Huang L, Zhou HY, Xie Z, Chen ZH, Wu YL (2013) Targeting mTOR to overcome epidermal growth factor receptor tyrosine kinase inhibitor resistance in non-small cell lung cancer cells. PLoS One 8:e69104
Xu L, Kikuchi E, Xu C, Ebi H, Ercan D, Cheng KA, Padera R, Engelman JA, Janne PA, Shapiro GI, Shimamura T, Wong KK (2012) Combined EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of lung cancers codriven by mutant EGFR containing T790M and MET. Cancer Res 72:3302–3311
Ono N, Yamazaki T, Tsukaguchi T, Fujii T, Sakata K, Suda A, Tsukuda T, Mio T, Ishii N, Kondoh O, Aoki Y (2013) Enhanced antitumor activity of erlotinib in combination with the Hsp90 inhibitor CH5164840 against non-small cell lung cancer. Cancer Sci 104:1346–1352
Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, Harada M, Akie K, Ogura S, Ishida T, Munakata M, Dosaka-Akita H, Isobe H, Nishimura M (2007) Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer 7:51
Tomizawa Y, Fujita Y, Tamura A, Shirai M, Shibata S, Kawabata T, Shibayama T, Fukai S, Kawahra M, Saito R (2010) Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 68:269–272
Beau-Faller M, Prim N, Ruppert A-M, Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet J-L, Rouquette I, de Crémoux P, Solassol J, de Fraipont F, Bièche I, Cayre A, Favre-Guillevin E, Tomasini P, Wislez M, Besse B, Legrain M, Voegeli A-C, Baudrin L, Morin F, Zalcman G, Quoix E, Blons H, Cadranel J (2014) Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 25:126–131
Sequist LV, Yang JC-H, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai C-M, Boyer M, Su W-C, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334
Conflict of interest
All authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, DH., Kim, Kp., Choi, CM. et al. EGFR–TKI is effective regardless of treatment timing in pulmonary adenocarcinoma with EGFR mutation. Cancer Chemother Pharmacol 75, 197–206 (2015). https://doi.org/10.1007/s00280-014-2631-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2631-5