Abstract
Purpose
To investigate the role of preoperative neutrophil-to-lymphocyte ratio (NLR) in prediction of response to first-line platinum-based chemotherapy and survival outcome in serous ovarian cancer (SOC) patients.
Methods
Clinicopathologic data were reviewed for patients with SOC treated with primary cytoreduction followed by platinum-based chemotherapy. The correlations of NLR value with clinicopathological features, clinical response to chemotherapy, and survival outcome were further explored.
Results
High preoperative NLR was significantly associated with advanced FIGO stage, histological grade, increased serum CA-125 level, and positive lymph node metastasis (P < 0.05, respectively). SOC patients in the third and fourth NLR quartile had significantly lower complete response rates compared to those in the first NLR quartile. In addition, survival analysis identified NLR as an independent prognostic factor for both PFS (HR 2.262, 95 % CI 1.342–3.811; P = 0.002) and OS (HR 3.254, 95 % CI 1.741–6.084; P < 0.001) in SOC patients.
Conclusions
Our findings indicated that high levels of preoperative NLR might be a potential biomarker of worse response to first-line platinum-based chemotherapy and poor clinical outcomes in patients with SOC. Further validation of this easily available parameter as a potential stratification tool in prospective studies should be encouraged.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) remains the most lethal gynecologic malignancy and one of the leading causes of cancer-related deaths in women worldwide [1]. High-grade serous cancer is the most common subtype, accounting for approximately 70 % of all cases of EOC [2]. Despite high rates of remission following radical surgery and platinum-based chemotherapy, the majority of patients will experience disease relapse at some point and ultimately drug resistance, resulting in a 5-year survival rate of only 19–28 % or even less [3]. Response to platinum or not has been proved to be a clinically useful proxy for predicting prognosis as well as guide for predicting future response to second-line chemotherapy [4]. Accordingly, identification of cancer biomarkers, in addition to common clinicopathological risk factors, to predict chemotherapy sensitivity and strengthen disease surveillance remains a major obstacle.
Inflammation plays a critical role in the development and progression of numerous cancers by upregulation of cytokines and inflammatory mediators, inhibition of apoptosis, induction of angiogenesis, stimulation of DNA damage, mediation of immunosuppression, and remodeling of the extracellular matrix [5, 6]. Recently, the neutrophil-to-lymphocyte ratio (NLR), an easily measured, reproducible, and inexpensive marker of systemic inflammation response, had been previously shown to serve as an independent prognostic marker for decreased survival in various cancer types, including colorectal cancer, breast cancer, gastric cancer, and soft tissue sarcoma [7–10]. Furthermore, several studies have indicated that an elevated pretreatment NLR may be useful as an adjunct in the evaluation of treatment response and disease recurrence [9, 11–13]. Although there were limited data regarding the potential prognostic significance of NLR in ovarian cancer [14–16], to the best of our knowledge, clinical studies in serous ovarian cancer (SOC) have yet to distinguish between the potential roles of NLR as a predictive biomarker of response to platinum-based chemotherapy.
In the present study, therefore, we sought to determine whether the preoperative NLR can be used as a prognostic marker for predicting response to chemotherapy and survival outcomes in patients with serous ovarian carcinoma.
Materials and methods
Study population and clinical data
This study was approved by the Institutional Review Board (IRB) of Tianjin Medical University Cancer Institute and Hospital. Written informed consent was obtained from all of the patients.
Medical records from patients diagnosed with SOC in our hospital between January 2009 and December 2010 were retrospectively reviewed. All patients were histologically confirmed and underwent cytoreductive surgery including para-aortic and pelvic lymph node dissection followed by platinum-based chemotherapy. Patients with second malignancies or multiple primary malignancies, hematological disease, inflammatory disease, hematology influenced drugs use, or missing preoperative complete blood cell count prior to surgery were excluded. In addition, patients were ineligible if they had undergone prior chemotherapy or radiotherapy.
Clinicopathological variables such as age, Eastern Cooperative Oncology Group (ECOG) performance status, surgical International Federation of Gynaecology and Obstetrics (FIGO) stage, histologic grade, residual tumor size, malignant ascites, lymph node metastasis, response to platinum-based chemotherapy, and preoperative leukocytes count were obtained retrospectively from the medical records. All blood routine was taken within 1 week before surgery, and the NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count; PLR (platelet-to-lymphocyte ratio) was defined as the absolute platelet count divided by the absolute lymphocyte count. Plasma quartile values of NLR, PLR, neutrophil, as well as platelet are demonstrated in Table 1.
Follow-up and evaluation
Prior to each cycle, patients were assessed clinically and radiological examinations were ordered if necessary. Thereafter, follow-up visits were scheduled every 3 months for 2 years, every 6 months for the next 3 years, and every 12 months thereafter. All patients were periodically followed until they died or until May 31, 2014.
Oncologic evaluation includes physical and clinical examination, and imaging of the chest, abdomen, and pelvis. The evaluation of the responses to first-line chemotherapy was evaluated according to RECIST criteria or the Gynecologic Cancer Intergroup (GCIG) CA-125 criteria [17, 18]. After the sixth cycle of chemotherapy, patients with no evidence of disease at clinical, sonographic, and radiological examination were defined as being in clinical complete response (CR).
Progression-free survival (PFS) was defined as the time from the chemotherapy initiation until disease progressed. Overall survival (OS) was defined as the interval between the date of chemotherapy initiation and the date of death or the most recent follow-up.
Statistical analysis
Patients were divided into equal quartiles according to the 25th, 50th, and 75th NLR percentile (i.e., the fourth or highest NLR quartile included the patients with the uppermost 25 % NLR values). The association between baseline clinicopathologic characteristics and NLR quartiles was evaluated by Pearson’s Chi-squared test. Logistic regression was used to analyze independent risk factors for predicting response to platinum-based chemotherapy. Univariate analysis of the different clinical factors associated with survival was carried out using Kaplan–Meier curves and compared by the log-rank test. Multivariable survival analysis was performed using Cox proportional hazards method. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19.0 (Chicago, IL, USA).
Results
Baseline characteristics
A total of one hundred and twenty-six patients with SOC who met the criteria were included in this study. The baseline characteristics of SOC patients sorted by their NLR quartiles are presented in Table 2. As depicted, high NLR levels were significantly correlated with advanced FIGO stage (P = 0.010), histological grade (P = 0.036), increased serum CA-125 level (P = 0.003), and positive lymph node metastasis (P = 0.014), whereas not with other clinicopathologic factors, including patient age, ECOG performance status, residual tumor size, and the presence of malignant ascites (P > 0.05, respectively).
Chemotherapeutic response
As for the response to first-line chemotherapy, 88 (69.8 %) patients obtained CR following platinum/taxane chemotherapy, eight (6.3 %) patients got partial response (PR), 21(16.7 %) patients had progressive disease (PD), and nine (7.1 %) patients had stable disease (SD).
The characteristics of clinicopathological factors affecting response to first-line platinum-based chemotherapy are shown in Table 3. Statistic data indicated that preoperative NLR (P = 0.005), patient age (P = 0.008), FIGO stage (P = 0.010), residual tumor size (P < 0.001), and serum CA-125 level (P = 0.038) were predictors of clinical response to treatment.
Furthermore, as shown in Table 4, multivariate logistic regression analysis suggested that NLR remained to be an independent factor associated with treatment response. Overall, patients in the third NLR quartile [odds ratio (OR) 4.646, 95 % confidence interval (CI) 1.299–9.837; P = 0.023] and fourth NLR quartile (OR 8.145, 95 % CI 2.520–14.235; P < 0.001) had significantly lower CR rates compared to patients in the first NLR quartile. Besides NLR, old age (OR 3.184, 95 % CI 1.677–5.598; P = 0.012), advanced FIGO stage (OR 4.220, 95 % CI 1.730–9.102; P = 0.014), and large residual tumor size (OR 5.929; 95 % CI 1.648–11.493; P = 0.004) were independently correlated with a lower probability of achieving CR.
Survival analysis
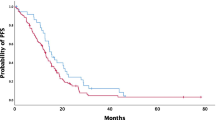
With a median follow-up time of 41.3 (range 3.3–70.4) months, 56.3 % (71/126) patients had experienced local or distant recurrence, and 47.6 % (60/126) had died as a result of disease progression, whereas the remaining patients were alive. Kaplan–Meier curves for PFS and OS according to quartiles of the NLR levels are shown in Fig. 1a, b. Pairwise log-rank test indicated significant differences between the first quartile compared with the second, third, and fourth quartiles (P < 0.05, respectively). Clinicopathological variables for prediction of prognosis were determined in univariate and multivariate Cox proportional models (Tables 5, 6).
Univariate analysis demonstrated that high NLR, old age, advanced FIGO stage, high tumor grade, large residual tumor size, malignant ascites, elevated CA-125 level, and platelets >380 × 109/L were unfavorable predictors for PFS (all P < 0.05).
Multivariate analysis identified high NLR, old age, advanced FIGO stage, and large residual tumor size as independent prognostic factors associated with poor PFS. Compared with patients in the first NLR quartile, the hazard ratio of PFS in the third and fourth quartile increased by 3.554 (P = 0.008) and 6.871 (P < 0.001), respectively.
For OS prediction, preoperative NLR, patient age, FIGO stage, residual tumor size, histologic grade, and serum CA-125 level were predictors confirmed by univariate analysis.
In multivariate analysis, only plasma NLR value within the third quartile (HR 5.302, 95 % CI 1.817–15.471; P = 0.002) and fourth quartile (HR 8.567, 95 % CI 2.808–26.136; P < 0.001), and large residual tumor size (HR 3.954, 95 % CI 1.994–7.838; P < 0.001) were independent prognostic indicators of unfavorable OS.
Discussion
A growing body of evidence highlights the importance of inflammation in the initiation, promotion, invasion, and metastasis of cancer [5]. During chronic inflammation, a wide array of intracellular signaling pathways are often deregulated, thereby leading to malignant transformation through the induction of genomic instability, damage of DNA, stimulation of cell proliferation, and promotion of angiogenesis [19]. Moreover, inflammatory mediators present in the tumor microenvironment, such as cytokines and interleukins, have been shown to be correlated with chemoresistance in several types of tumor, including ovarian cancer [20, 21].
The neutrophil-to-lymphocyte ratio (NLR), an emerging marker of host inflammation, has been demonstrated to be a prognosticator for various malignancies. With respect to ovarian cancer, several studies have provided evidence for the association between NLR and prognosis after surgical resection. An early study conducted by Cho et al. reported that the NLR level is significantly elevated in ovarian cancer cases compared to those with benign gynecologic diseases or healthy controls. In that study, they also found that the NLR can identify CA-125-negative cases and predict poor outcome [14]. Recently, Williams et al. [15] retrospectively evaluated 519 women with ovarian carcinoma and showed that elevated NLR signals more aggressive disease, correlates with risk factors such as family history, and predicts poor survival.
In the present work, we not only validated the prognostic impact of NLR levels on survival outcome, but also clearly demonstrated that an elevated NLR level was associated with worse pathologic features such as advanced tumor stage for ovarian serous carcinoma. The PFS and OS were significantly longer among patients within the first NLR quartile than those within the second, third, and fourth quartile. In addition to preoperative NLR, inflammatory markers such as PLR, neutrophils, and platelets count have been proved to be of prognostic value as well [22, 23]. When these markers were considered in our study, only platelet count within the highest quartile (platelet > 380 × 109/L) was significantly associated unfavorable PFS; however, this difference lost statistical significance in multivariate analysis. Although the molecular basis of the relationship between elevated NLR levels and poor clinical outcome in cancer patients has not been fully elucidated, several possible explanations have been postulated. First, oncogenic changes and tumor growth can induce tissue inflammation and hence increase the NLR levels [24]. Second, the high NLR reflects an elevated neutrophil level. Several lines of evidence suggest that neutrophils may promote tumor development via remodeling extracellular matrix, releasing pro-angiogenic factors, and suppressing lymphocyte activity [19, 25]. Third, elevated NLR reflects a relative lymphopenia. Lymphocytes are known to play a critical role in cancer immune-surveillance, which inhibits proliferation and metastatic activity of tumor cells [26].
In line with previous studies, which have shown that patients with high level of NLR have a worse response to chemotherapy [9, 11], positive correlation of preoperative NLR with chemotherapeutic response was demonstrated in the current study as well. The CR rates in different NLR quartiles were 90.3, 71.9, 68.8, and 48.4 %, respectively, and the difference was statistically significant (P = 0.005). In addition, we also found that patient age, FIGO stage, serum CA-125 value, as well as residual tumor size were independent predictors for the chance of achieving a CR to treatment. More importantly, multivariate logistic regression analysis confirmed that NLR remained to be an independent factor associated with treatment response. Patients in the third and fourth NLR quartile had significantly lower CR rates compared to patients in the first NLR quartile, implying that high level of NLR can be used alone or in combination with other markers to identify patients who are more susceptible to chemoresistance.
However, there are a few limitations to this study. First, NLR is known to be a non-specific marker of inflammation, and it is also possible that the presence of other systemic diseases could influence the NLR level in the peripheral blood. Second, our study is limited by its retrospective nature and a relatively small sample size. Finally, we did not calculate the NLR during or after chemotherapy and therefore cannot analyze whether dynamic change of NLR presents a predictive value.
Conclusions
In summary, our initial experience confirms the potential utility of preoperative NLR levels as an independent prognostic marker in SOC patients. Moreover, elevated preoperative NLR may be a promising indicator for worse chemotherapeutic response and platinum resistance in patients with SOC. However, further large-scale prospective multicentre studies should be encouraged to confirm and extend these findings.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics 2013. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Kobel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, Seidman JD, Gilks CB (2010) Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 29(3):203–211. doi:10.1097/PGP.0b013e3181c042b6
Alkema NG, Tomar T, van der Zee AG, Everts M, Meersma GJ, Hollema H, de Jong S, van Vugt MA, Wisman GB (2014) Checkpoint kinase 2 (Chk2) supports sensitivity to platinum-based treatment in high grade serous ovarian cancer. Gynecol Oncol 133(3):591–598. doi:10.1016/j.ygyno.2014.03.557
Cohen S, Mosig R, Moshier E, Pereira E, Rahaman J, Prasad-Hayes M, Halpert R, Billaud JN, Dottino P, Martignetti JA (2014) Interferon regulatory factor 1 is an independent predictor of platinum resistance and survival in high-grade serous ovarian carcinoma. Gynecol Oncol. doi:10.1016/j.ygyno.2014.06.025
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. doi:10.1038/nature01322
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91(3):181–184. doi:10.1002/jso.20329
Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S (2013) Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 30(1):432. doi:10.1007/s12032-012-0432-4
Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, Shim CN, Lee H, Shin SK, Lee SK, Lee YC (2014) Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. doi:10.1007/s10120-013-0330-2
Choi ES, Kim HS, Han I (2014) Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol 21(3):778–785. doi:10.1245/s10434-013-3418-3
Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S, Chiba T (2014) Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 3(2):406–415. doi:10.1002/cam4.204
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18(12):3362–3369. doi:10.1245/s10434-011-1754-8
Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M (2010) Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol 184(3):873–878. doi:10.1016/j.juro.2010.05.028
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K (2009) Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 58(1):15–23. doi:10.1007/s00262-008-0516-3
Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW (2014) Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 132(3):542–550. doi:10.1016/j.ygyno.2014.01.026
Thavaramara T, Phaloprakarn C, Tangjitgamol S, Manusirivithaya S (2011) Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J Med Assoc Thail 94(7):871–877
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Vergote I, Rustin GJ, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MK, Friedlander M, Jakobsen A, Vermorken JB (2000) Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). Gynecologic Cancer Intergroup. J Natl Cancer Inst 92(18):1534–1535
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899. doi:10.1016/j.cell.2010.01.025
Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, Li LZ (2010) Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett 295(1):110–123. doi:10.1016/j.canlet.2010.02.019
Ferrandina G, Lauriola L, Zannoni GF, Fagotti A, Fanfani F, Legge F, Maggiano N, Gessi M, Mancuso S, Ranelletti FO, Scambia G (2002) Increased cyclooxygenase-2 (COX-2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann Oncol 13(8):1205–1211
Asher V, Lee J, Innamaa A, Bali A (2011) Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 13(7):499–503. doi:10.1007/s12094-011-0687-9
Banerjee S, Rustin G, Paul J, Williams C, Pledge S, Gabra H, Skailes G, Lamont A, Hindley A, Goss G, Gilby E, Hogg M, Harper P, Kipps E, Lewsley LA, Hall M, Vasey P, Kaye SB (2013) A multicenter, randomized trial of flat dosing versus intrapatient dose escalation of single-agent carboplatin as first-line chemotherapy for advanced ovarian cancer: an SGCTG (SCOTROC 4) and ANZGOG study on behalf of GCIG. Ann Oncol 24(3):679–687. doi:10.1093/annonc/mds494
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 6(4):283–287. doi:10.1023/B:AGEN.0000029415.62384.ba
Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90:1–50. doi:10.1016/S0065-2776(06)90001-7
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, P., Xu, Y. et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother Pharmacol 75, 255–262 (2015). https://doi.org/10.1007/s00280-014-2622-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2622-6