Abstract
Purpose
Dinaciclib is a novel selective inhibitor of cyclin-dependent kinase (CDK)1, CDK2, CDK5, and CDK9. We conducted a phase I study to investigate the effects of dinaciclib when administered with rituximab.
Methods
In this phase I nonrandomized dose-escalation 3 + 3 trial, patients with relapsed/refractory chronic lymphocytic leukemia (CLL) were treated with dinaciclib and rituximab. Dinaciclib was administered intravenously (IV) over 2 h on days 1, 8 and 15 in cycles 2–13 (28-day cycles). Rituximab 375 mg/m2 was administered IV on days 1, 8, 15 and 22 in cycle 1 (28-day cycle) and on day 1 during cycle 3–13. Rituximab was not administered in cycle 2. Rituximab and dinaciclib were given alone in cycles 1 and 2, respectively, and in combination in cycles 3–13. Primary objectives included determination of the recommended phase II dose of dinaciclib and evaluation of pharmacokinetics (PK) when administered with rituximab.
Results
Five patients completed the study due to early termination. All presented with drug-related adverse events (AEs), but no dose-limiting toxicities were observed. The most commonly observed toxicities included hematological, digestive and metabolic AEs. However, no tumor lysis syndrome has been reported in the study. Four patients achieved stable disease, and one patient achieved complete response according to 2008 iwCLL criteria at cycle 3. PK samples were collected from 5 patients, and no obvious interaction between dinaciclib and rituximab was observed.
Conclusions
Limited data from this study shows dinaciclib in combination with rituximab was well tolerated and revealed encouraging clinical activity in relapsed/refractory CLL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fludarabine, cyclophosphamide and rituximab are now considered standard treatment of physically fit chronic lymphocytic leukemia (CLL) patients in first line and are also often used in relapse [1–4]. Patients with purine analogs refractory CLL, defined as failure to achieve partial response (PR) by NCI-WG criteria or progressive disease during treatment or within 6 months of the last treatment, have a dismal prognosis. These patients may benefit from treatment involving monoclonal antibodies. However, even with such options, responses are short-lived [5–7], emphasizing the need for novel therapeutic options in relapsed/refractory CLL patients [8].
Dinaciclib (MK-7965, formerly SCH 727965) is a novel, potent, small molecule inhibitor of cyclin-dependent kinase (CDK) [9]. This molecule selectively inhibits CDK1, CDK2, CDK5, and CDK9 at IC50 values in the 1–4 nM range. In murine xenograft models, dinaciclib has a superior therapeutic activity with an improved therapeutic index compared with the pan-CDK inhibitor flavopiridol [10–12]. CDK are serine/threonine kinases that regulate progression through the cell cycle and are bound by various cyclins that activate the CDKs in specific phases of the cell cycle. CDK1 participates in S, G2 and M-phase progression [13–16]. CDK2 plays a critical role in regulating cell cycle progression through the G1/S transition and S and G2 phases. Altogether CDK1 and CDK2 regulate cell cycle progression and checkpoint control. CDK5 is involved in cytoskeletal regulation. Together with CDK7 and CDK8, CDK9 has been shown to be involved in the phosphorylation of the RNA polymerase II carboxyl terminal domain, participating to cellular transcription. Mcl-1 is a short half-life protein which is particularly sensitive to RNAPII inhibition following dinaciclib treatment [17–19]. In vitro studies have shown dinaciclib to be a potent inducer of apoptosis in tumor cell lines [20–22] and an effective inhibitor of tumor growth in murine xenograft models of human cancers, using tissue fragments of patient-derived xenografts grown in mice [10, 23–25].
Rituximab is a genetically engineered chimeric murine/human IgG1 kappa anti-CD20 monoclonal antibody with high affinity for the CD20 surface antigen that is selectively expressed on B lymphocytes. Rituximab binding to B cells results in sustained B cell depletion and has significantly improved clinical outcome in patients with CD20-positive B-cell malignancies, including CLL [26–28]. The mechanisms of antitumor effect of rituximab include antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and direct antibody-mediated apoptosis [29]. In vitro studies have shown that rituximab-mediated inhibition of cell survival signaling pathways and downregulation of anti-apoptotic Bcl2 family members can sensitize resistant tumor cells to apoptosis by chemotherapeutic agents [30]. Furthermore, reduction of anti-apoptotic protein Mcl-1 by siRNA knockdown has been shown to enhance rituximab-mediated apoptosis in CLL patients [31]. The development of Mcl-1 targeted therapies may enhance the therapeutic benefit of rituximab. Altogether these results suggest the rationale for the clinical development of the dinaciclib plus anti-CD20 monoclonal antibody therapeutic combination.
Several in vitro studies show the benefit to combine rituximab and dinaciclib. Among anti-apoptotic Bcl2 family members, Mcl-1, when downregulated, enhances rituximab-mediated apoptosis and CDC in CLL patient cells [30, 31]. A role for Mcl-1 in rituximab resistance in CLL is also supported by a significantly higher Mcl-1/Bax expression ratio in nonresponsive rituximab-treated patients as compared to responsive patients [31]. In addition, dinaciclib exhibits a CDK9 inhibitory activity, thus blocking CDK9-dependent phosphorylation of RNAPII, which is required for transcriptional elongation [16, 32]. Mcl-1 is a short anti-apoptotic protein, particularly sensitive to RNAPII inhibition, further emphasizing the benefit of combining anti-CD20 rituximab antibody and dinaciclib. Patients had previously received anti-CD20 treatment, including rituximab in 40 % and ofatumumab in 20 % of cases. The possibility to retreat patients with anti-CD20 antibodies in combination with dinaciclib enhances therapeutic options in this heavily pretreated cohort of patients.
Phase I clinical studies of dinaciclib as single agent have demonstrated an acceptable safety profile [33, 34]. Most common adverse events (AEs) across all trials are neutropenia, leukopenia, anemia, thrombocytopenia, diarrhea, nausea, vomiting, fatigue, increased liver enzymes, hyperglycemia, hypocalcemia and hypotension.
In the P04629 study, dinaciclib as single agent was administered on a weekly schedule in patients with advanced solid tumors and hematological malignancies. Stable disease (SD) control was seen in some solid tumors and lymphoma patients; however, the most promising clinical activity was seen in CLL. Overall response rate was 58.3 % (according to NCI Working Group Response Criteria, 28/48, all PR) [9]. Subjects assigned to dose regimens containing 14 mg/m2 (14, 10 → 14 and 7 → 10 → 14 mg/m2 combined) had a response rate of 66.7 % (22/33, all PR). Based on safety and efficacy data, the 7 → 10 → 14 mg/m2 was chosen as the recommended phase II dose.
In this trial, subjects with relapsed or refractory CLL/small lymphocytic lymphoma (SLL) received dinaciclib in combination with rituximab. A modified 3 + 3 design was used to establish the MTD of dinaciclib when given in combination with rituximab. Rituximab 375 mg/m2 IV was administered on day 1, 8, 15 and 22 in cycle 1 (28 day cycle) and on day 1 in cycles 3–13 (28 day cycles). Rituximab was not administered in cycle 2.
We report our initial clinical experience with dinaciclib in combination with rituximab in relapsed and refractory CLL patients, along with pharmacokinetic (PK) studies.
Patients and methods
Eligibility criteria
This nonrandomized open-label phase I dose-escalation trial was designed to investigate the effect of dinaciclib when administered with rituximab. Patients with confirmed CLL or SLL, as defined by the 2008 International Workshop on CLL (iwCLL) criteria, who were 18 years of age or older, and had received at least one prior therapy including either fludarabine or equivalent nucleoside analog, were eligible to enroll in the trial. Patients had to have Eastern Cooperative Oncology Group performance status of 0, 1, or 2, as well as adequate organ function and laboratory parameters. Patients who received treatment with a cytochrome P450 3A4 (CYP3A4) inhibitor or inducer within 1 week prior to enrollment, or any chemotherapy or biological therapy within 4 weeks prior to enrollment, were excluded from the study. Patients who previously received an allogeneic bone marrow transplant or any prior treatment with a CDK inhibitor were excluded. Concomitant use of other drugs that are inhibitors or inducers of CYP3A4 were prohibited while patients were enrolled in the study. Patients who had active autoimmune anemia or idiopathic thrombocytopenic purpura unless stable were also excluded from the study. Blood sample for FISH testing has been performed at screening using probes for del17 (p13.1), del13 (q14), del11 (q22.3) and trisomy 12. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki concerning written informed consent and the protection of rights of human subjects participating in biomedical research. Before study initiation, the clinical study protocol, any amendments and the written informed consent forms were reviewed and approved by an independent review board at each study site. Each subject had to provide written informed consent before undergoing any study-related activities.
Study design and objectives
This was a phase 1b nonrandomized open-label multisite trial, designed to determine the tolerable dose of dinaciclib therapy in combination with rituximab in patients with relapsed and refractory CLL (ClinicalTrials.gov: NCT01650727; Study P07974). The study originally consisted of two parts. The first part was to determine the MTD of dinaciclib when given in combination with rituximab, and the second part was to determine the overall response rate (ORR) of the combination therapy. Dinaciclib was administered intravenously (IV) over 2 h on days 1, 8 and 15 in cycles 2–13 (28-day cycles) (Level−1). Dinaciclib was to be administered at a dose of 7 mg/m2 as a 2 h infusion on day 1, escalated to 10 mg/m2 beginning with day 8 and continuing thereafter (Level-1). Escalating doses of 7 → 10 → 14 mg/m2 in cycle 1 were administered and 14 mg/m2 in cycle 2 and thereafter (Level 2). Rituximab was administered IV at 375 mg/m2 on day 1, 8, 15 and 22 in cycle 1 and on day 1 during cycle 3 through 13. Rituximab was not administered in cycle 2. Both dinaciclib and rituximab drugs were only combined at cycle 3, allowing the first two cycles to reduce tumor burden and prevent tumor lysis syndrome (TLS) (Table 1). In order to minimize the incidence of TLS, patients started at a lower dose of dinaciclib before increasing either to 10 or 14 mg/m2.
All premedication and supportive care could be used per institutional standard. Erythropoietin and G-CSF were not allowed for the first three cycles to ensure proper dose-limiting toxicity assessment. Laboratory TLS work-up was performed prior to the start of infusion and at 3 and 5 h after the start of dinaciclib infusion at cycle 2.
Safety and response assessments
All patients enrolled in the study who received study medication were included in the safety analysis. AEs were monitored throughout the study and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.1) and characterized according to their relationship to study medication by the investigator. AEs were collected from the date of enrollment until the date of the post-study visit. Safety assessments also included clinical laboratory (hematology and chemistry) measurements, 12-lead electrocardiograms, vital signs performed at screening and on prespecified times throughout treatment. Disease response was assessed according to the 2008 iwCLL criteria. ORR (ORR = complete response [CR] + PR) is defined as the proportion of patients whose best response is confirmed PR or CR as per the 2008 iwCLL. Computed tomography scans of the chest, abdomen and pelvis were obtained every 12 weeks after the patient began treatment (cycle 1 day 1) until completion of study treatment or until disease progression was assessed.
Pharmacokinetic assessments
Plasma dinaciclib concentrations were determined immediately prior to the start of the infusion (pre-dose) on day 1 of cycle 3 and at predefined intervals after the start of the infusion. Timed blood PK samples were collected at 1, 2, 3 and 5 h after the initiation of the infusion. The data and time of each sample collection were recorded.
Statistical analyses
Patient demographic data and baseline characteristics were summarized using descriptive statistics. Serious AEs (SAEs) were reviewed in real-time basis, and periodic reviews of AEs were performed during the trial. The number of patients reporting any AEs, the occurrence of specific AEs and discontinuation due to AEs were tabulated and summarized using descriptive statistics. Summary statistics (means, standard deviations) were calculated for the concentration data at each sampling time and the derived PK parameters. The log-transformed PK parameters were analyzed using an analysis of variance model extracting the effects due to treatment, period, sequence and patient. The point estimate of the relative exposure to dinaciclib, when administered with or without rituximab, was calculated along with the corresponding 90 % confidence interval. Figures were generated using Sigma Plot 10 (Systat Software Inc., San Jose, CA) and S-PLUS 8 (TIBCO Software Inc., Palo Alto, CA).
Results
Patient characteristics
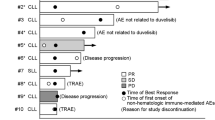
Five patients were enrolled and treated in the study. Their data were evaluable for PK parameters. Patient demographics and baseline characteristics are summarized in Table 2. The majority of patients enrolled in the trial were male (60 %), and the median age was 74 years [range 63–80]. Eighty percent of patients were older than 65 years, 40 % of patients had RAI stage 3 or 4, and 40 % had Bulky disease. Median number of prior treatments received was 2 [range 1–6]. Eighty percent had previously received fludarabine and 60 % monoclonal antibody-based treatment. According to cytogenetic data, none of the patients had del17p.
Safety and tolerability
Treatment-related AEs are shown in Table 3. All patients experienced at least one AE (Table 4). Treatment-related AEs were reported in all patients. Among these, four patients presented with SAEs. Drug-related AEs as determined by the investigator were observed in all patients, with two of them presenting with SAEs exclusively observed in the cohort dinaciclib 7–10–10 mg/m2. None of the patients had to discontinue the study because of drug-related AEs. Table 3 summarizes treatment-related AEs that occurred in at least one patient following treatment combining dinaciclib and rituximab. The most frequent treatment-related AEs were hematological, including anemia, leukopenia, neutropenia and thrombocytopenia, with a frequency ≥40 %. Among digestive AEs, diarrhea was the most commonly observed. In this heavily pretreated cohort of patients, asthenia and pneumonia were also frequent. No TLS occurred in patients. One patient experienced acute kidney injury during cycle 7 with increase in serum creatinine from 83 µmol/L at baseline to 160 µmol/L. This was resolved with supportive care measures. The patient continued study treatment once serum creatinine returned to the baseline value. Altogether, the combination of dinaciclib and rituximab was well tolerated.
Clinical outcome and efficacy
Mean duration of exposure to dinaciclib was 127.4 days. Patients treated in the dinaciclib 7–10–10 mg/m2 cohort were treated for 105 days [range 35–189] whereas patients in the 7–10–14 mg/m2 cohort received 30.3 days [range 21–42]. Shortly after the second cohort patients started treatment, this study was stopped prematurely due to administrative reasons. As a result, analysis of safety and efficacy data for 7–10–14 mg/m2 cohort was for a shorter period of time. A listing of efficacy responses is provided in Table 5. One patient achieved CR according to iwCLL criteria. At cycle 3, 4 patients (80 %) achieved SD. Among patients, two were still in SD at cycle 6, while two had progressive disease. One patient was lost to follow-up.
Pharmacokinetics
Owing to sparse PK samples collected in the study, the systemic exposure area under the curve (AUC) as well as half-life (t 1/2) for the patients could not be reported. However, the maximum plasma concentration (C max) and time to C max (T max) values were comparable, given variability with those observed in a previous phase I study in patients with CLL at similar dose levels (ClinicalTrials.gov: NCT00871663). The individual plasma concentration–time profiles for dinaciclib are presented in Fig. 1. Dinaciclib demonstrated similar PK profiles and exposure when administered alone or in combination with rituximab. The PK analysis from previous phase I studies showed that dinaciclib, as a single agent, was rapidly eliminated with t 1/2 ranging from 2.31 to 2.95 h.
Discussion
In this study, dinaciclib was administered in combination with rituximab in refractory CLL patients. Overall, combination treatment was well tolerated and observed AEs did not differ from known AEs when dinaciclib is used as a single agent. The most frequent treatment-related AEs were hematological with a frequency ≥40 %. Digestive AEs, including diarrhea, were also frequent. We did not observe any discontinuation of the study because of drug-related AEs. No TLS occurred. As in other phases I trials using dinaciclib [33–37], patients were treated according to a dose-titration exploration. Altogether our data suggest that administration of dinaciclib and rituximab is a feasible combination with a manageable safety profile.
PK data from clinical studies show peak drug plasma concentrations were achieved at 1–2 h and dinaciclib exhibited rapid distribution and elimination phases (t 1/2 ranging from 1.5 to 3.3 h) [38]. Clearance was dose independent with no drug accumulation in plasma. Dinaciclib demonstrated similar PK profiles and exposure when administered alone or in combination with rituximab. However, due to limited PK samples, the systemic exposure AUC as well as t 1/2 for the patients could not be reported in our study.
Due to early termination of enrollment, planned efficacy analysis was not performed in our study. Despite a limited number of enrolled patients, one patient achieved CR in the lowest cohort. Among four patients (80 %) in SD at cycle 3, two of them were still in SD at cycle 6. None of the five treated patients presented with unfavorable del17p cytogenetics. Despite preliminary data suggesting that dinaciclib is effective in high-risk CLL, according to chromosomal abnormalities [33, 34, 36], no conclusion could be drawn in the current study.
This phase I study investigated the effects of a novel selective CDK inhibitor, dinaciclib when administered with rituximab. Five relapsed/refractory CLL patients received rituximab alone in cycle 1, dinaciclib alone in cycle 2 and the combination of both, starting cycle 3. As shown in our study, the combination of dinaciclib and rituximab demonstrated efficacy, with four patients achieving SD at cycle 3, but of short duration. Furthermore, this association seems to be well tolerated with no TLS reported. Altogether, this preliminary results warrant further studies combining dinaciclib to other anti-CD20 antibodies such as ofatumumab in relapsed/refractory CLL.
References
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer M et al (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376:1164–1174
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al (2005) Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 23:4079–4088
Tam CS, O’Brien S, Weirda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 112:975–980
Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, Burger J, Koller C, Lerner S, Kantarjian H, Wierda WG (2011) Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 117:3016–3024
Cuthill K, Devereux S (2013) How I treat patients with relapsed chronic lymphocytic leukaemia. Br J Haematol 163:423–435
Cortelezzi AM, Sciume M, Liberati AM, Vincenti D, Cuneo A, Laurenti L et al (2014) Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia 28:642–648
Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, Sargent R, Burger JA, Farrajoli A (2013) Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 31:584–591
Rodriguez-Vicente AE, Diaz MG, Hernandez-Rivas JM (2013) Chronic lymphocytic leukemia: a clinical and molecular heterogenous disease. Cancer Genet 206:49–62
Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou YM, Statkevich P, Yao SL, Bannerji R (2013) A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 11:259
Parry D, Guzi T, Shanahan F, Davis N, Prabjavalkar D, Wiswell D et al (2010) Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther 9:2344–2353
Blachly JS, Byrd JC (2013) Emerging drug profile: cyclin-dependent kinase inhibitors. Leuk Lymphoma 54:2133–2143
Bose P, Simmons GL, Grant S (2013) Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs 22:723–738
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166
Morgan DO (1995) Principles of CDK regulation. Nature 374:131–134
Sausville EA (2002) Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med 8(4 Suppl):S32–S37
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24:1770–1783
Lim S, Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093
Gallorini M, Cataldi A, di Giacomo V (2012) Cyclin-dependent kinase modulators and cancer therapy. BioDrugs 26:377–391
Canavese M, Santo L, Raje N (2012) Cyclin dependent kinases in cancer: potential for therapeutic intervention. Cancer Biol Ther 13:451–457
Johnson AJ, Smith LL, Wagner AJ, Hessler J, Flynn JM, Jones JA, Zhang X et al (2010) Dinaciclib (SCH727965) is a novel cyclin dependent kinase inhibitor that promotes selective apoptosis in CLL cells and abrogates the protective effects of microenvironment cytokines. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 971
Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer J, Altiok S, Pledger WJ (2011) The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells. Mol Cancer Ther 10:1018–1027
Desai BM, Villanueva J, Nguyen TT, Lioni M, Xiao M, Kong J et al (2013) The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS ONE 8:e59588
Abdullah C, Wang X, Becker D (2011) Expression analysis and molecular targeting of cyclin-dependent kinases in advanced melanoma. Cell Cycle 10:977–988
Fu W, Sharma SS, Ma L, Chu B, Bui MM, Reed D, Pledger WJ (2013) Apoptosis of osteosarcoma cultures by the combination of the cyclin-dependent kinase inhibitor SCH727965 and a heat shock protein 90 inhibitor. Cell Death Dis 4:e566
Feldmann G, Mishra A, Bisht S, Karikari C, Garrido-Laguna I, Rasheed Z et al (2011) Cyclin-dependent kinase inhibitor dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther 12:598–609
Robak T (2012) Rituximab for chronic lymphocytic leukemia. Expert Opin Biol Ther 12:503–515
Lepretre S, Jager U, Janssens A, Leblond V, Nikitin E, Robak T, Wendtner CM (2012) The value of rituximab for the treatment of fludarabine-refractory chronic lymphocytic leukemia: a systematic review and qualitative analysis of the literature. Leuk Lymphoma 53:820–829
Griffin MM, Morley N (2013) Rituximab in the treatment of non-Hodgkin’s lymphoma—a critical evaluation of randomized controlled trials. Expert Opin Biol Ther 13:803–811
Weiner GJ (2010) Rituximab: mechanism of action. Semin Hematol 47:115–123
Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC (2002) The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 99:1038–1043
Hussain SR, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, Lucas DM, Byrd JC (2007) Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res 13:2144–2150
Burger K, Muhl B, Rohrmoser M, Coordes B, Heidemann M, Kellner M et al (2013) Cyclin-dependent kinase 9 links RNA polymerase II transcription to processing of ribosomal RNA. J Biol Chem 288:21173–21183
Flynn JM, Jones JA, Andritsos L, Blum KA, Johnson AJ, Hessler J, et al (2010) Update on the phase I study of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): confirmation of clinical activity and feasibility of long-term administration. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 1396
Flynn JM, Jones JA, Andritsos L, Blum KA, Johnson AJ, Hessler J, et al (2011) Phase I study of the CDK inhibitor dinaciclib (SCH 727965) in patients (pts) with relapsed/refractory CLL. Presented at the 2011 ASCO annual meeting, 3–7, June 2011, Chicago, IL, Abstract 6623
Baiocchi RA, Flynn JM, Jones JA, Blum KA, Hofmeister CC, Poon J, et al (2010) Early evidence of anti-lymphoma activity of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in heavily pre-treated low grade lymphoma and diffuse large cell lymphoma patients. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 3966
Flynn JM, Andritsos LA, Jones JA, Johnson AJ, Maddocks K, Wiley E, et al (2013) Dinaciclib (SCH 727965) is a novel cyclin-dependent kinase (CDK) inhibitor that exhibits activity in patients with relapsed or refractory chronic lymphocytic leukemia (CLL). Presented at the 55th ASH annual meeting and exposition, 7–10, December 2013, New Orleans, LA, Abstract 871
Sadowska M, Muvarak N, Lapidus RG, Sausville EA, Bannerji R, Gojo I (2010) Single agent activity of the cyclin-dependent kinase (CDK) inhibitor dinaciclib (SCH 727965) in acute myeloid and lymphoid leukemia cells. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 3981
Mita MM, Mita AC, Moseley J, Poon J, Small KA, Jou Y, et al (2011) A phase I study of the CDK inhibitor dinaciclib (SCH 727965) administered every 3 weeks in patients (pts) with advanced malignancies: final results. Presented at the 2011 ASCO annual meeting, 3–7, June 2011, Chicago, IL, Abstract 3080
Acknowledgments
The authors would like to thank Dr. Jérôme Couturier (Institut Curie, Paris, France), Pr. Frédéric Davi (Hôpital Pitié-Salpétrière, Paris, France), Dr. Claire Mathiot (Institut Curie, Paris, France), Dr. Vincent Servois (Institut Curie, Paris, France), for their contributions to biological and radiological assessments of the study and Kristen Lewis of Merck & Co., Inc., Whitehouse Station, NJ for editorial assistance. The study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ).
Conflict of interest
CF, MG, CE, MZ, MPS and CLT have no conflicts of interest to disclose. KS, EI, NS, DZ and HZ are current or former employees of Merck & Co., Inc., Whitehouse Station, NJ and may own stock/stock options in the company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabre, C., Gobbi, M., Ezzili, C. et al. Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol 74, 1057–1064 (2014). https://doi.org/10.1007/s00280-014-2583-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2583-9