Abstract
Purpose
Hypoxia-inducible factor-1 (HIF-1) facilitates the adaptation of normal and tumor tissues to oxygen deprivation. HIF-1 is frequently overexpressed in cancer cells, where it is involved in the upregulation of many genes necessary for survival. EZN-2968 is an antisense oligodeoxynucleotide that specifically targets HIF-1α, one of the subunits of HIF-1. We conducted a trial of EZN-2968 in patients with refractory solid tumors to evaluate antitumor response and to measure modulation of HIF-1α mRNA and protein levels as well as HIF-1 target genes.
Methods
Adult patients with refractory advanced solid tumors were administered EZN-2968 as a 2-h IV infusion at a dose of 18 mg/kg once a week for three consecutive weeks followed by 3-week off; in a 6-week cycle. Tumor biopsies and dynamic contrast enhanced MRI (DCE-MRI) were performed at baseline and after the third dose.
Results
Ten patients were enrolled, of whom all were evaluable for response; one patient with a duodenal neuroendocrine tumor had prolonged stabilization of disease (24 weeks). Reduction in HIF-1α mRNA levels compared to baseline was demonstrated in 4 of 6 patients with paired tumor biopsies. Reductions in levels of HIF-1α protein and mRNA levels of some target genes were observed in two patients. Quantitative analysis of DCE-MRI from two patients revealed changes in K trans and k ep. The trial was closed prematurely when the sponsor suspended development of this agent.
Conclusion
This trial provides preliminary proof of concept for modulation of HIF-1α mRNA and protein expression and target genes in tumor biopsies following the administration of EZN-2968.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
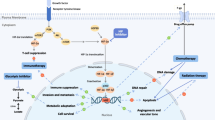
Hypoxia-inducible factor-1 (HIF-1), a transcription factor, facilitates tissue adaptation to oxygen deprivation by inducing the expression of genes necessary for cell survival [1]. HIF-1 directly activates the transcription of pro-angiogenic factors including VEGF. High concentrations of HIF-1 are associated with poor prognosis and resistance to therapy in patients with numerous solid tumors, making HIF-1 an attractive target for cancer therapeutics [1–4]. HIF-1 is a heterodimer consisting of the HIF-1α subunit, inducible by tissue hypoxia, and the HIF-1β subunit, which is constitutively expressed. Levels of HIF-1 can be modulated by targeting HIF-1α transcription, protein translation, DNA binding, or protein degradation [5].
Various inhibitors of HIF-1α have been described, but they lack specificity [5, 6]. Antisense oligonucleotides are an attractive therapeutic strategy because they can inhibit mRNA expression specifically [7, 8]. However, one of the main limitations is the difficulty of intracellular delivery to the tissue of interest. Synthetic locked nucleic acid (LNA) antisense oligodeoxynucleotides are a new class of nucleic acid analog that have demonstrated higher affinity, specificity, and resistance to degradation compared to other oligonucleotides [9, 10].
EZN-2968 is an LNA antisense oligodeoxynucleotide that has been demonstrated to hybridize with HIF-1α mRNA and block HIF-1α protein expression in preclinical models [11]. Tumor growth inhibition was observed in xenograft models [11]. Safety and a recommended Phase 2 dose was determined, and preliminary evidence of activity observed in two Phase 1 clinical trials of EZN-2968. Stable disease with evidence of tumor shrinkage was reported in 15 % and 33 % of patients, respectively [12, 13].
Based on these promising preclinical and clinical results, we conducted a pilot trial with the primary objective of measuring modulation of HIF-1α mRNA in tumor biopsies pre- and post-administration of EZN-2968. Secondary objectives were to evaluate the safety and antitumor efficacy of EZN-2968 and to measure modulation of HIF-1α protein levels and HIF-1 target gene mRNA levels in tumor biopsies before and after treatment.
Patients and methods
Eligibility criteria
Patients (age ≥18 years) were eligible if they had pathologically confirmed solid tumor that had progressed following standard therapy; an Eastern Cooperative Oncology Group performance status ≤2; and adequate organ function defined as absolute neutrophil count ≥1,500/μL, platelets ≥100,000/μL, total bilirubin ≤1.5 × the upper limit of normal (ULN), aspartate aminotransferase and/or alanine aminotransferase <2.5 × ULN, creatinine <1.5 × ULN, and 24-h urine protein level <500 mg (if urine protein/creatinine ratio >1). Because preclinical biodistribution studies of EZN-2968 documented prolonged and preferential retention in the liver [11], we initially restricted enrollment to patients with advanced solid tumors where the liver involvement represented the predominant disease burden to optimize our ability to demonstrate target modulation and provide clinical benefit (2 patients). We performed tumor biopsies of liver lesions to assess target modulation. Liver-predominant disease was defined as intrahepatic disease of at least 6 cm in longest diameter (either in one single lesion or as a sum of multiple lesions) and fewer than six malignant lesions outside the liver with no single lesion greater than 3 cm. However, because several clinical responses were observed with EZN-2968 in tumors outside the liver in other studies, the eligibility criteria were subsequently modified to allow patients with widespread disease to enroll (8 patients).
Patients were required to have disease amenable to biopsy and be willing to undergo paired tumor biopsies. Previous anticancer therapy must have been completed at least 4 weeks prior to enrollment. Patients with brain metastases within the past 3 months, or who were pregnant or lactating were not eligible. This trial was conducted under a National Cancer Institute (NCI)-sponsored IND with Institutional Review Board approval. The protocol design and conduct followed all applicable regulations, guidance, and local policies (ClinicalTrials.gov Identifier: NCT01120288).
Trial design
This was an open-label, single arm, pilot trial of EZN-2968 in patients with refractory solid malignancies. EZN-2968 was administered as a 2-h IV infusion at a dose of 18 mg/kg once a week for three consecutive weeks followed by 3-week off, in a 6-week cycle [13]. EZN-2968 was supplied by the Division of Cancer Treatment and Diagnosis, NCI, under a collaborative agreement with Enzon Pharmaceuticals. Adverse events were graded according to NCI Common Toxicity Criteria version 3.0. Toxicities had to resolve to ≤grade 2 (hematologic) and ≤grade 1 (non-hematologic) prior to proceeding with the next dose. The next cycle could be delayed for a maximum of 2 weeks to allow for resolution of toxicities. In case of ≥grade 3 toxicity, the dose was reduced to the next lower dose level (12 mg/kg then 8 mg/kg).
Dynamic contrast enhanced MRI (DCE-MRI) was performed at baseline prior to EZN-2968 administration and within 2 days after the third dose using standard methods (described in the Supplementary Data, online only). Tumor biopsies were obtained before EZN-2968 administration on cycle 1 day 1 and after the third dose during cycle 1. All tumor biopsy samples were obtained from metastatic sites by experienced interventional radiologists.
A patient was considered evaluable to assess the primary objective if he/she had paired tumor biopsy specimens available for analysis. Inability to obtain tissue after a reasonable attempt would not have precluded treatment, and the patient would have remained eligible for all other translational components including imaging and the clinical endpoints of response and safety. However, the patient would have been replaced for the purposes of statistical analysis.
Safety and efficacy evaluations
History and physical examination and complete blood counts with differential and serum chemistries were performed at baseline and repeated weekly on days of treatment. Radiographic evaluation was performed at baseline and every two cycles to assess tumor response based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [14].
Pharmacodynamic evaluations
RNA extraction and real-time PCR analysis of HIF-1α and target genes
Methods are provided in the Supplementary Data section (online only). Values are expressed as percent change relative to the pretreatment sample for each patient. Assessment of changes in the expression to select HIF-1α target genes, VEGF, PDK-1, CAIX and GLUT-1, was performed in patients from whom sufficient RNA from paired tumor biopsies was available (Fig. 1c). Gene expression was measured relative to baseline levels.
HIF-1α mRNA, HIF-1α protein, and HIF-1α target gene mRNA levels were determined in paired tumor biopsies collected at baseline (pre-dose) and following the administration of EZN-2968. a HIF-1α and B2M mRNA copy numbers were determined in comparison with a plasmid DNA standard curve; HIF-1α mRNA copy number was then normalized to mRNA copy number for B2M. Percentage indicates overall change from baseline. b HIF-1α protein levels in 5 patients; percentage indicates overall change from baseline. *Pre-dose biopsy <LLQ; +post-dose biopsy <LLQ. c RT-PCR for HIF-1α target genes VEGF, CAIX, GLUT-1, and PDK-1. Results expressed as mRNA fold change in EZN-2968 treatment samples relative to baseline, arbitrarily considered equal to 1
HIF-1α immunoassay
Methods for specimen collection to preserve HIF-1α in 18-gauge tumor needle biopsies and an assay to quantitatively measure HIF-1α levels in human tissue biopsy were validated (Supplementary Data section, online only) [15].
Statistical analysis
This study was conducted as a single-stage pilot trial to determine modulation of HIF-1α mRNA following treatment with EZN-2968 as measured by RT-PCR. For purposes of sample size determination, the primary endpoint was the proportion of patients in whom expression of HIF-1α mRNA in a tumor biopsy decreased by 50 % compared to baseline. The enrollment goal was a total of 20 evaluable patients with paired biopsies and measurable HIF-1α protein levels.
Results
Patient demographics
A total of 10 patients were enrolled (Table 1); the study was closed prematurely when further clinical development of EZN-2968 was suspended by the pharmaceutical sponsor. Median number of treatment cycles was 2 (1–4 cycles). One patient with low-grade neuroendocrine malignancy (Patient #2) had stable disease following 4 cycles of therapy (24 weeks); this patient chose to come off study for personal reasons. A 78-year-old patient with malignant pleural mesothelioma (Patient #9) had stable disease for 8 weeks when he came off treatment due to grade 2 elevated creatinine levels, which subsequently resolved. The remaining patients had progressive disease during either the first or second cycle of therapy.
Toxicity
EZN-2968 was generally well tolerated without significant toxicity (Table 2). A 77-year-old male patient with multiple liver metastases who had grade 1 AST elevation at baseline demonstrated worsening of AST to grade 3 after the second dose of study drug during cycle 1. Treatment was held until resolution to a grade 1 toxicity level was demonstrated one week later; the dose was reduced to 12 mg/kg. However, the patient was subsequently taken off study due to grade 3 AST elevation and a CT scan that showed disease progression. One other patient experienced a treatment-related grade 2 ALT elevation.
A 79-year-old male patient with underlying chronic renal insufficiency developed a grade 2 elevation in creatinine which returned to baseline (grade 1). The most common grade 2 toxicity was lymphopenia (4 patients). One patient had a grade 2 infusion reaction that was attributed to complement activation, a known class effect of phosphorothioate-linked antisense oligodeoxynucleotides [16]; this toxicity resolved within a few hours after antihistamine and steroid treatment. This side effect developed during cycle 2, week 3 of treatment, and the remainder of the infusion was discontinued. Because of disease progression, the patient was taken off study prior to the next cycle.
HIF-1α mRNA and protein levels
Six patients had paired pre- and post-dose biopsies for the assessment of HIF-1α mRNA levels (Patients #2, 3, 6, 7, 9, and 10; Fig. 1a); five patients had paired biopsies for the assessment of HIF-1α protein levels (Patients #1, 2, 3, 6, and 7; Fig. 1b). Five of the patients assessed had decreased HIF-1α mRNA copy numbers ranging from −7 to −94 % from baseline. Three patients had decreased HIF-1α protein levels following EZN-2968 treatment, of which two (Patients #1 and #7) had a greater than 50 % decrease.
Four patients (Patients #2, 3, 6, and 7) had both HIF-1α protein and mRNA copy number assessed. In agreement with the expected pharmacodynamic changes following EZN-2968 administration, Patients #3 and 7 had decrease in levels of both HIF-1α protein and mRNA. In contrast, Patient #6 had increase in level of HIF-1α protein and mRNA, while Patient #2 had a discordant 113 % increase in HIF-1α protein and 30 % decrease in HIF-1α mRNA.
Expression of HIF-1α target genes
Assessment of HIF-1α target gene expression (Fig. 1c; Supplemental Table S2) was performed in the six paired biopsies. In the limited number of patient samples available for analysis, there were no clear patterns of decreased expression as a result of EZN-2968 therapy, or overall associations with changes in either HIF-1α protein or mRNA levels.
DCE-MRI
Evaluable DCE-MRI data were available for only two patients. The first, a patient with rectal adenocarcinoma (Patient #1), had a 48 % decrease in K trans value and a 33 % decrease in k ep value between baseline and post-treatment DCE-MRI. The second, a patient with Hurthle cell thyroid carcinoma (Patient #3), had 35 and 14 % increases in K trans and k ep values, respectively. Both patients had progressive disease after one cycle (6 weeks).
Discussion
Conclusive evidence demonstrating modulation of HIF-1α mRNA, protein and target genes in tumor tissue following EZN-2968 treatment was not obtained in this study due to the small numbers of patients entered as a consequence of the study’s premature closure. Only one patient met the predefined study criteria of a greater than 50 % decrease in HIF-1α mRNA; however, sufficient tumor biopsy material was not available to perform protein analysis for this patient. The lack of association could also be due to the inherent heterogeneity in HIF-1α expression and protein levels in human tumors. Reports in the literature demonstrate clusters of HIF-1α-positive cells at the edge of tumor margins, around necrotic regions and areas of neovascularization using immunohistochemical analysis [17, 18]. Even though our group has developed a validated assay to measure HIF-1α protein levels that provided reliable and reproducible results [15], quantifying HIF-1α protein in human tumor biopsy samples remained challenging.
Two patients had a decrease in levels of both HIF-1α mRNA and protein following EZN-2968 treatment, yet there was no corresponding decrease in the expression of the four HIF-1-targeted genes evaluated. This may be due to HIF-1α-independent regulation of gene expression (e.g., HIF-2α-dependent or HIF-independent), or it may be cell- or tumor-type dependent. Interestingly, the expression of VEGF, which is commonly considered a HIF-1α and HIF-2α target gene [19], was unchanged in all of the samples examined. GLUT-1, known to be targeted by both HIF-1α and HIF-2α, was only decreased in 2 patients [19]. IC50 values for the HIF-2α isoform, with which EZN-2968 has a 3-base-pair mismatch, were 5 times higher in all cell lines tested compared to IC50 values for HIF-1α, supporting the relative selectivity of EZN-2968 for HIF-1α [11].
EZN-2968 was well tolerated at the dose and schedule described; most of the toxicities were grade 1 or 2, and there were no unexpected toxicities. Hepatic and renal toxicities have been reported previously with the administration of phosphorothioate antisense oligonucleotides, as have complement activation and prolongation of PTT due to interaction with complement and coagulation factor [16]. We did not observe any cases of PTT prolongation, but one patient had a grade 2 infusion reaction attributed to complement activation.
Only one case of grade 1 anemia was observed, which suggests that EZN-2968 did not suppress erythropoietin production in the kidney, despite the fact that erythropoietin is a target gene of HIF-2α. There were no new cases of hypertension in this study of EZN-2968, consistent with the lack of effect on VEGF expression observed in our analysis. However, given the small number of patients on the trial and the fact that interrogation of the VEGF pathway was not part of the study, it is difficult to draw definitive conclusions about the antiangiogenic effects of EZN-2968 in humans [20].
This study was initially designed to enroll patients in whom liver lesions represented the predominant disease burden to optimize our ability to demonstrate target modulation in tumor biopsy samples. This was based on preclinical data that documented prolonged and preferential retention of EZN-2968 in the liver, kidney, and lymph nodes, while drug concentrations in colon, bone marrow, and lungs were below detectable limits [11]. Two patients were enrolled with liver-predominant disease as defined per protocol; the remaining eight patients had widespread metastatic disease. There were no differences in clinical response between these two groups of patients or across various sites of metastases. This could be due to the small number of patients in either group, lack of overall activity of the agent, or no difference in delivery of agent to the various sites of disease.
One of the critical considerations surrounding the successful development of anticancer oligonucleotides is demonstrating their ability to specifically target the RNA of interest within tumor cells. This study was designed to measure changes in HIF-1α mRNA and protein in tumor biopsies in patients with advanced solid tumors. A validated assay was developed to measure modulation of HIF-1α in human samples; however, statistically significant modulation of target was not demonstrated due to the small sample size and the heterogeneous nature of the target. Trials such as this one which evaluate pharmacodynamic endpoints as well as modulation of target-dependent gene expression can inform the clinical development of oligonucleotide-based cancer therapies.
References
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G (2002) Overexpression of hypoxia-inducible factor 1α is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 8:1831–1837
Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S et al (2003) Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res 23:4721–4727
Swinson DEB, Jones JL, Cox G, Richardson D, Harris AL, O’Byrne KJ (2004) Hypoxia-inducible factor-1α in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer 111:43–50
Hu Y, Liu J, Huang H (2013) Recent agents targeting HIF-1 alpha for cancer therapy. J Cell Biochem 114:498–509
Melillo G (2007) Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev 26:341–352
Aboul-Fadl T (2005) Antisense oligonucleotides: the state of the art. Curr Med Chem 12:2193–2214
Vester B, Wengel J (2004) LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry 43:13233–13241
Raunak, Ravindra Babu B, Sorensen MD, Parmar VS, Harrit NH, Wengel J (2004) Oligodeoxynucleotides containing alpha-L-ribo configured LNA-type C-aryl nucleotides. Org Biomol Chem 2:80–89
Nimesh S, Gupta N, Chandra R (2011) Cationic polymer based nanocarriers for delivery of therapeutic nucleic acids. J Biomed Nanotechnol 7:504–520
Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, Albaek C, Schroder H, Orum H (2008) A RNA antagonist of hypoxia-inducible factor-1 alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther 7:3598–3608
Patnaik A, Chiorean EG, Tolcher A, Papadopoulos K, Beeram M, Kee D, Waddell M, Gilles E, Buchbinder A (2009) EZN-2968, a novel hypoxia-inducible factor-1 (HIF-1) messenger ribonucleic acid (mRNA) antagonist: results of a phase I, pharmacokinetic (PK), dose-escalation study of daily administration in patients (pts) with advanced malignancies. J Clin Oncol 27:2564
Cohen RB, Olszanski A, Figueroa J, Hurwitz H, Lokiec FM, Rezaï K, Berkowitz N, Buchbinder A (2011) Down-modulation of messenger ribonucleic acid (mRNA) by EZN-2968, an hypoxia-inducible factor-1(HIF-1α) mRNA antagonist, administered in adult patients with advanced solid tumors [abstract]. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research, Orlando, Florida. AACR, Philadelphia (PA), abstract nr LB-407, 2–6 April 2011
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Park SR, Kinders RJ, Khin S, Hollingshead M, Parchment RE, Tomaszewski JE, Doroshow JH (2012) Validation and fitness testing of a quantitative immunoassay for HIF-1 alpha in biopsy specimens [abstract]. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research, Chicago, Illinois. AACR, Philadelphia (PA), abstract nr 3616, 31 March–4 April 2012
Levin AA (1999) A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta 1489:69–84
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW (1999) Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res 59:5830–5835
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157:411–421
Keith B, Johnson RS, Simon MC (2012) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9–22
Jeong W, Doroshow JH, Kummar S (2013) United States Food and Drug Administration approved oral kinase inhibitors for the treatment of malignancies. Curr Probl Cancer 37:110–144
Acknowledgments
We thank Drs. Yvonne, A. Evrard, and Andrea Voth for editorial assistance. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, W., Rapisarda, A., Park, S.R. et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother Pharmacol 73, 343–348 (2014). https://doi.org/10.1007/s00280-013-2362-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2362-z